The pheromone-induced nuclear accumulation of the Fus3 MAPK in yeast depends on its phosphorylation state and on Dig1 and Dig2
- PMID: 17963515
- PMCID: PMC2219999
- DOI: 10.1186/1471-2121-8-44
The pheromone-induced nuclear accumulation of the Fus3 MAPK in yeast depends on its phosphorylation state and on Dig1 and Dig2
Abstract
Background: Like mammalian MAP kinases, the mating-specific Fus3 MAPK of yeast accumulates in the nuclei of stimulated cells. Because Fus3 does not appear to be subjected to active nucleo-cytoplasmic transport, it is not clear how its activation by mating pheromone effects the observed change in its localization. One possibility is that the activation of Fus3 changes its affinity for nuclear and cytoplasmic tethers.
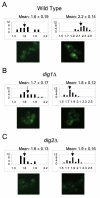
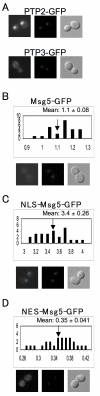
Results: Dig1, Dig2, and Ste12 are nuclear proteins that interact with Fus3. We found that the pheromone-induced nuclear accumulation of a Fus3-GFP reporter is reduced in cells lacking Dig1 or Dig2, whereas Fus3T180AY182A-GFP localization was unaffected by the absence of these proteins. This suggests that Dig1 and Dig2 contribute to the retention of phosphorylated Fus3 in the nucleus. Moreover, overexpression of Ste12 caused the hyper-accumulation of Fus3-GFP (but not Fus3T180AY182A-GFP) in the nuclei of pheromone-treated cells, suggesting that Ste12 also plays a role in the nuclear retention of phosphorylated Fus3, either by directly interacting with it or by transcribing genes whose protein products are Fus3 tethers. We have previously reported that overexpression of the Msg5 phosphatase inhibits the nuclear localization of Fus3. Here we show that this effect depends on the phosphatase activity of Msg5, and provide evidence that both nuclear and cytoplasmic Msg5 can affect the localization of Fus3.
Conclusion: Our data are consistent with a model in which the pheromone-induced phosphorylation of Fus3 increases its affinity for nuclear tethers, which contributes to its nuclear accumulation and is antagonized by Msg5.
Figures

Similar articles
-
Two novel targets of the MAP kinase Kss1 are negative regulators of invasive growth in the yeast Saccharomyces cerevisiae.Genes Dev. 1996 Nov 15;10(22):2831-48. doi: 10.1101/gad.10.22.2831. Genes Dev. 1996. PMID: 8918885
-
Effect of the pheromone-responsive G(alpha) and phosphatase proteins of Saccharomyces cerevisiae on the subcellular localization of the Fus3 mitogen-activated protein kinase.Mol Cell Biol. 2003 Feb;23(4):1135-50. doi: 10.1128/MCB.23.4.1135-1150.2003. Mol Cell Biol. 2003. PMID: 12556475 Free PMC article.
-
Regulation of the mating pheromone and invasive growth responses in yeast by two MAP kinase substrates.Curr Biol. 1997 Apr 1;7(4):228-38. doi: 10.1016/s0960-9822(06)00118-7. Curr Biol. 1997. PMID: 9094309
-
Fus3-triggered Tec1 degradation modulates mating transcriptional output during the pheromone response.Mol Syst Biol. 2008;4:212. doi: 10.1038/msb.2008.47. Epub 2008 Aug 5. Mol Syst Biol. 2008. PMID: 18682702 Free PMC article.
-
Mitogen-activated protein kinase (MAPK) dynamics determine cell fate in the yeast mating response.J Biol Chem. 2017 Dec 15;292(50):20354-20361. doi: 10.1074/jbc.AC117.000548. Epub 2017 Nov 9. J Biol Chem. 2017. PMID: 29123025 Free PMC article.
Cited by
-
Communicate and Fuse: How Filamentous Fungi Establish and Maintain an Interconnected Mycelial Network.Front Microbiol. 2019 Mar 29;10:619. doi: 10.3389/fmicb.2019.00619. eCollection 2019. Front Microbiol. 2019. PMID: 31001214 Free PMC article. Review.
-
Substrate-dependent control of MAPK phosphorylation in vivo.Mol Syst Biol. 2011 Feb 1;7:467. doi: 10.1038/msb.2010.121. Mol Syst Biol. 2011. PMID: 21283143 Free PMC article.
-
The transcription factor PstSTE12 is required for virulence of Puccinia striiformis f. sp. tritici.Mol Plant Pathol. 2018 Apr;19(4):961-974. doi: 10.1111/mpp.12582. Epub 2017 Sep 25. Mol Plant Pathol. 2018. PMID: 28710879 Free PMC article.
-
MAPKs in development: insights from Dictyostelium signaling pathways.Biomol Concepts. 2011 Apr 1;2(1-2):39-46. doi: 10.1515/BMC.2011.004. Biomol Concepts. 2011. PMID: 21666837 Free PMC article.
-
Regulation of Cell-to-Cell Communication and Cell Wall Integrity by a Network of MAP Kinase Pathways and Transcription Factors in Neurospora crassa.Genetics. 2018 Jun;209(2):489-506. doi: 10.1534/genetics.118.300904. Epub 2018 Apr 20. Genetics. 2018. PMID: 29678830 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

