Synthesis, nicotinic acetylcholine receptor binding, and pharmacological properties of 3'-(substituted phenyl)deschloroepibatidine analogs
- PMID: 17964169
- PMCID: PMC2374917
- DOI: 10.1016/j.bmc.2007.10.027
Synthesis, nicotinic acetylcholine receptor binding, and pharmacological properties of 3'-(substituted phenyl)deschloroepibatidine analogs
Abstract
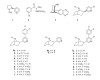
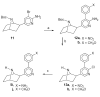
A series of 3'-(substituted phenyl)deschloroepibatidine analogs (5a-j) were synthesized. The alpha4beta2( *) and alpha7 nicotinic acetylcholine receptor (nAChR) binding properties and functional activity in the tail-flick, hot-plate, locomotor, and body temperature tests in mice of 5a-j were compared to those of the nAChR agonist, nicotine (1), epibatidine (4), and deschloroepibatidine (13), the partial agonist, varenicline (3), and the antagonist 2'-fluoro-3'-(substituted phenyl)deschloroepibatidine analogs (7a-j). Unlike epibatidine and deschloroepibatidine, which are potent agonists in the tail-flick test, 5a-k show no or very low antinociceptive activity in the tail-flick or hot-plate test. However, they are potent antagonists in nicotine-induced antinociception in the tail-flick test, but weaker than the corresponding 2'-fluoro-3'-(substituted phenyl)deschloroepibatidines.
Figures

Similar articles
-
Synthesis, nicotinic acetylcholine receptor binding, and antinociceptive properties of 2'-fluoro-3'-(substituted phenyl)deschloroepibatidine analogues. Novel nicotinic antagonist.J Med Chem. 2004 Aug 26;47(18):4588-94. doi: 10.1021/jm040078g. J Med Chem. 2004. PMID: 15317468
-
Synthesis, Nicotinic Acetylcholine Receptor Binding, and in Vitro and in Vivo Pharmacological Properties of 2'-Fluoro-(substituted thiophenyl)deschloroepibatidine Analogues.ACS Chem Neurosci. 2017 Jan 18;8(1):115-127. doi: 10.1021/acschemneuro.6b00252. Epub 2016 Oct 20. ACS Chem Neurosci. 2017. PMID: 27726337
-
Synthesis, nicotinic acetylcholine receptor binding, and antinociceptive properties of 2'-fluoro-3'-(substituted pyridinyl)-7-deschloroepibatidine analogues.J Med Chem. 2014 Feb 13;57(3):836-48. doi: 10.1021/jm401602p. Epub 2014 Jan 28. J Med Chem. 2014. PMID: 24428686 Free PMC article.
-
Synthesis, nicotinic acetylcholine receptor binding, and antinociceptive properties of 3'-substituted deschloroepibatidine analogues. Novel nicotinic antagonists.J Med Chem. 2005 Feb 24;48(4):1221-8. doi: 10.1021/jm040160b. J Med Chem. 2005. PMID: 15715488
-
Epibatidine structure-activity relationships.Bioorg Med Chem Lett. 2004 Apr 19;14(8):1889-96. doi: 10.1016/j.bmcl.2004.02.007. Bioorg Med Chem Lett. 2004. PMID: 15050621 Review.
Cited by
-
Competitive docking model for prediction of the human nicotinic acetylcholine receptor α7 binding of tobacco constituents.Oncotarget. 2018 Feb 8;9(24):16899-16916. doi: 10.18632/oncotarget.24458. eCollection 2018 Mar 30. Oncotarget. 2018. PMID: 29682193 Free PMC article.
-
EPIBATIDINE ANALOGS SYNTHESIZED FOR CHARACTERIZATION OF NICOTINIC PHARMACOPHORES-A REVIEW.Heterocycles. 2009;79:99-120. doi: 10.3987/rev-08-sr(d)1. Heterocycles. 2009. PMID: 25530665 Free PMC article.
-
The effects of nicotine, varenicline, and cytisine on schedule-controlled responding in mice: differences in α4β2 nicotinic receptor activation.Eur J Pharmacol. 2011 Mar 1;654(1):47-52. doi: 10.1016/j.ejphar.2010.12.003. Epub 2010 Dec 21. Eur J Pharmacol. 2011. PMID: 21172344 Free PMC article.
-
The antinociceptive effects of nicotinic partial agonists varenicline and sazetidine-A in murine acute and tonic pain models.J Pharmacol Exp Ther. 2012 Sep;342(3):742-9. doi: 10.1124/jpet.112.194506. Epub 2012 Jun 7. J Pharmacol Exp Ther. 2012. PMID: 22678099 Free PMC article.
-
Synthesis, nicotinic acetylcholine receptor binding, and antinociceptive properties of 3'-(substituted phenyl)epibatidine analogues. Nicotinic partial agonists.J Nat Prod. 2010 Mar 26;73(3):306-12. doi: 10.1021/np9006124. J Nat Prod. 2010. PMID: 20038125 Free PMC article.
References
-
- Ezzati M, Lopez AD. Lancet. 2003;362:847–852. - PubMed
-
- Henningfield JE, Fant RV, Buchhalter AR, Stitzer ML. CA Cancer J Clin. 2005;55:281–299. - PubMed
-
- Keating GM, Siddiqui MAA. CNS Drugs. 2006;20:945–960. - PubMed
-
- Niaura R, Jones C, Kirkpatrick P. Nat Rev Drug Discov. 2006;5:537–538. - PubMed
-
- Carroll FI. Bioorg & Med Chem Ltrs. 2004;14:1889–1896. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

