Pyrin activates the ASC pyroptosome in response to engagement by autoinflammatory PSTPIP1 mutants
- PMID: 17964261
- PMCID: PMC2719761
- DOI: 10.1016/j.molcel.2007.08.029
Pyrin activates the ASC pyroptosome in response to engagement by autoinflammatory PSTPIP1 mutants
Abstract
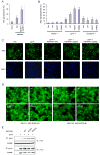
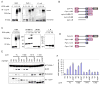
The molecular mechanism by which mutations in the cytoskeleton-organizing protein PSTPIP1 cause the autoinflammatory PAPA syndrome is still elusive. Here, we demonstrate that PSTPIP1 requires the familial Mediterranean fever protein pyrin to assemble the ASC pyroptosome, a molecular platform that recruits and activates caspase-1. We provide evidence that pyrin is a cytosolic receptor for PSTPIP1. Pyrin exists as a homotrimer in an autoinhibited state due to intramolecular interactions between its pyrin domain (PYD) and B-box. Ligation by PSTPIP1, which is also a homotrimer, activates pyrin by unmasking its PYD, thereby allowing it to interact with ASC and facilitate ASC oligomerization into an active ASC pyroptosome. Because of their high binding affinity to pyrin's B-box, PAPA-associated PSTPIP1 mutants were found to be more effective than WT PSTPIP1 in inducing pyrin activation. Therefore, constitutive ligation and activation of pyrin by mutant PSTPIP1 proteins explain the autoinflammatory phenotype seen in PAPA syndrome.
Figures





References
-
- Badour K, Zhang J, Shi F, McGavin MK, Rampersad V, Hardy LA, Field D, Siminovitch KA. The Wiskott-Aldrich syndrome protein acts downstream of CD2 and the CD2AP and PSTPIP1 adaptors to promote formation of the immunological synapse. Immunity. 2003;18:141–154. - PubMed
-
- Bao Q, Shi Y. Apoptosome: a platform for the activation of initiator caspases. Cell Death Differ. 2007;14:56–65. - PubMed
-
- Cao T, Borden KL, Freemont PS, Etkin LD. Involvement of the rfp tripartite motif in protein–protein interactions and subcellular distribution. J Cell Sci. 1997;110:1563–1571. - PubMed
-
- Centola M, Wood G, Frucht DM, Galon J, Aringer M, Farrell C, Kingma DW, Horwitz ME, Mansfield E, Holland SM, et al. The gene for familial Mediterranean fever, MEFV, is expressed in early leukocyte development and is regulated in response to inflammatory mediators. Blood. 2000;95:3223–3231. - PubMed
-
- Chae JJ, Centola M, Aksentijevich I, Dutra A, Tran M, Wood G, Nagaraju K, Kingma DW, Liu PP, Kastner DL. Isolation, genomic organization, and expression analysis of the mouse and rat homologs of MEFV, the gene for familial mediterranean fever. Mamm Genome. 2000;11:428–435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

