Registering histologic and MR images of prostate for image-based cancer detection
- PMID: 17964460
- PMCID: PMC2483248
- DOI: 10.1016/j.acra.2007.07.018
Registering histologic and MR images of prostate for image-based cancer detection
Abstract
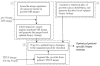
Rationale and objectives: Needle biopsy is currently the only way to confirm prostate cancer. To increase prostate cancer diagnostic rate, needles are expected to be deployed at suspicious cancer locations. High-contrast magnetic resonance (MR) imaging provides a powerful tool for detecting suspicious cancerous tissues. To do this, MR appearances of cancerous tissue should be characterized and learned from a sufficient number of prostate MR images with known cancer information. However, ground-truth cancer information is only available in histologic images. Therefore it is necessary to warp ground-truth cancerous regions in histological images to MR images by a registration procedure. The objective of this article is to develop a registration technique for aligning histological and MR images of the same prostate.
Material and methods: Five pairs of histological and T2-weighted MR images of radical prostatectomy specimens are collected. For each pair, registration is guided by two sets of correspondences that can be reliably established on prostate boundaries and internal salient bloblike structures of histologic and MR images.
Results: Our developed registration method can accurately register histologic and MR images. It yields results comparable to manual registration, in terms of landmark distance and volume overlap. It also outperforms both affine registration and boundary-guided registration methods.
Conclusions: We have developed a novel method for deformable registration of histologic and MR images of the same prostate. Besides the collection of ground-truth cancer information in MR images, the method has other potential applications. An automatic, accurate registration of histologic and MR images actually builds a bridge between in vivo anatomical information and ex vivo pathologic information, which is valuable for various clinical studies.
Figures







Similar articles
-
Registering histological and MR images of prostate for image-based cancer detection.Med Image Comput Comput Assist Interv. 2006;9(Pt 2):620-8. doi: 10.1007/11866763_76. Med Image Comput Comput Assist Interv. 2006. PMID: 17354824
-
Elastic registration of prostate MR images based on estimation of deformation states.Med Image Anal. 2015 Apr;21(1):87-103. doi: 10.1016/j.media.2014.12.007. Epub 2015 Jan 8. Med Image Anal. 2015. PMID: 25624044
-
Semiautomatic registration of digital histopathology images to in vivo MR images in molded and unmolded prostates.J Magn Reson Imaging. 2014 May;39(5):1223-9. doi: 10.1002/jmri.24287. Epub 2013 Oct 17. J Magn Reson Imaging. 2014. PMID: 24136783 Free PMC article.
-
Quantification of liver fat with magnetic resonance imaging.Magn Reson Imaging Clin N Am. 2010 Aug;18(3):337-57, ix. doi: 10.1016/j.mric.2010.08.013. Magn Reson Imaging Clin N Am. 2010. PMID: 21094444 Free PMC article. Review.
-
Interactive Feature Space Explorer© for multi-modal magnetic resonance imaging.Magn Reson Imaging. 2015 Jul;33(6):804-15. doi: 10.1016/j.mri.2015.03.007. Epub 2015 Apr 11. Magn Reson Imaging. 2015. PMID: 25868623 Free PMC article. Review.
Cited by
-
Quantitative identification of magnetic resonance imaging features of prostate cancer response following laser ablation and radical prostatectomy.J Med Imaging (Bellingham). 2014 Oct;1(3):035001. doi: 10.1117/1.JMI.1.3.035001. Epub 2014 Oct 27. J Med Imaging (Bellingham). 2014. PMID: 26158070 Free PMC article.
-
Contrast enhanced-magnetic resonance imaging as a surrogate to map verteporfin delivery in photodynamic therapy.J Biomed Opt. 2013 Dec;18(12):120504. doi: 10.1117/1.JBO.18.12.120504. J Biomed Opt. 2013. PMID: 24365954 Free PMC article.
-
Decision support system for localizing prostate cancer based on multiparametric magnetic resonance imaging.Med Phys. 2012 Jul;39(7):4093-103. doi: 10.1118/1.4722753. Med Phys. 2012. PMID: 22830742 Free PMC article.
-
Optimum slicing of radical prostatectomy specimens for correlation between histopathology and medical images.Int J Comput Assist Radiol Surg. 2010 Sep;5(5):471-87. doi: 10.1007/s11548-010-0405-z. Epub 2010 Feb 24. Int J Comput Assist Radiol Surg. 2010. PMID: 20180036 Review.
-
A PET/CT Directed, 3D Ultrasound-Guided Biopsy System for Prostate Cancer.Prostate Cancer Imaging (2011). 2011;6363:100-108. doi: 10.1007/978-3-642-23944-1_11. Prostate Cancer Imaging (2011). 2011. PMID: 26866061 Free PMC article.
References
-
- Christens-Barry WA, Partin AW. Quantitative Grading of Tissue and Nuclei in Prostate Cancer for Prognosis Prediction. Johns Hopkins Apl Technical Digest. 1997;18:226–233.
-
- Rifkin M, Zerhouni E, Gatsonis C, Quint L, Paushter D, Epstein J, Hamper U, Walsh P, McNeil B. Comparison of magnetic resonance imaging and ultrasonography in staging early prostate cancer. The New England Journal of Medicine. 1990;323:621–626. - PubMed
-
- Ikonen S, Kaerkkaeinen P, Kivisaari L, Salo JO, Taari K, Vehmas T, Tervahartiala P, Rannikko S. Magnetic Resonance Imaging of Clinically Localized Prostatic Cancer. JOURNAL OF UROLOGY. 1998;159:915–919. - PubMed
-
- Madabhushi A, Feldman M, Metaxas DN, Chute D, Tomaszewski J. A Novel Stochastic Combination of 3D Texture Features for Automated Segmentation of Prostatic Adenocarcinoma from High Resolution MRI. presented at MICCAI; 2003.
-
- Chan I, Wells W, Mulkern RV, Haker S, Zhang J, Zou KH, Maier SE, Tempany CM. Detection of prostate cancer by integration of line-scan diffusion, T2-mapping and T2-weighted magnetic resonance imaging; a multichannel statistical classifier. Med Phys. 2003;30:2390–8. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

