Pro-NGF, sortilin, and p75NTR: potential mediators of injury-induced apoptosis in the mouse dorsal root ganglion
- PMID: 17964555
- PMCID: PMC2156563
- DOI: 10.1016/j.brainres.2007.09.051
Pro-NGF, sortilin, and p75NTR: potential mediators of injury-induced apoptosis in the mouse dorsal root ganglion
Abstract
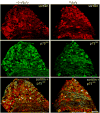
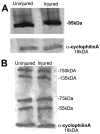
The nerve growth factor precursor (pro-NGF) may function as a death-inducing ligand that mediates its apoptotic effects via p75NTR. Pro-NGF-induced apoptosis is postulated to be dependent upon membrane expression of the sortilin receptor, which interacts with p75NTR to promote a high-affinity binding site for pro-NGF. Here, we explore the expression of pro-NGF, sortilin and p75NTR in the mouse lumbar dorsal root ganglion (DRG) to understand the potential for this trimeric signaling complex to function in injury-induced neuronal death of DRG neurons. Our results reveal the expression of all 3 components within the DRG and that a subpopulation of neurons coexpresses sortilin and p75NTR. Following sciatic nerve transection, the expression of these proteins appears insensitive to injury; however, the majority of small p75NTR-sortilin coexpressing neurons are lost 25 days after sciatic nerve transection. These results propose pro-NGF-induced, p75NTR-sortilin-mediated neuronal death as a critical aspect of nerve injury-induced death in the DRG.
Figures

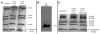

References
-
- Chen Y, Dicou E, Djakiew D. Characterization of nerve growth factor precursor protein expression in rat round spermatids and the trophic effects of nerve growth factor in the maintenance of Sertoli cell viability. Molecular and Cellular Endocrinology. 1997;127:129–136. - PubMed
-
- Christianson JA, Ryals JM, McCarson KE, Wright DE. Beneficial actions of neurotrophin treatment on diabetes-induced hypoalgesia in mice. J Pain. 2003;4:493–504. - PubMed
-
- Christianson JA, Ryals JM, McCarson KE, Wright DE. Beneficial actions of neurotrophin treatment on diabetes-induced hypoalgesia in mice. J Pain. 2003;4:493–504. - PubMed
-
- Crockett DP, Harris SL, Egger MD. Neurotrophin receptor (p75) in the trigeminal thalamus of the rat: development, response to injury, transient vibrissa-related patterning, and retrograde transport. The Anatomical Record. 2000;259:446–460. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

