"Upstream markers" provide for early identification of patients at high risk for myocardial necrosis and adverse outcomes
- PMID: 17964560
- PMCID: PMC3569496
- DOI: 10.1016/j.cca.2007.09.023
"Upstream markers" provide for early identification of patients at high risk for myocardial necrosis and adverse outcomes
Abstract
Background: For patients presenting with acute coronary syndrome (ACS) to the emergency department, early identification of those that are at high risk for subsequent myocardial necrosis or adverse outcomes would allow earlier or more aggressive treatment. We determined if a panel of biomarkers can be used to identify high risk patients.
Methods: A cohort (84 females/132 males) from our 1996 ACS study population that had EDTA specimens stored (-70 degrees C) was selected and the earliest available specimen was analyzed for 11 biomarkers (IL-6, IL-8, MCP-1, VEGF, L-selectin, P-selectin, E-selectin, ICAM-1, VCAM-1, NT-proBNP, cTnT). These data were linked to the existing cTnI and health outcome databases for this population. ROC curve analysis for myocardial necrosis (i.e., peak cTnI >0.04 microg/l) identified 3 candidate biomarkers. These 3 biomarkers were applied together to generate a panel test (2 of the 3 biomarkers increased for a positive result) and assessed for its ability to identify patients at risk for myocardial necrosis and the combined endpoint of death, myocardial infarction (MI) and heart failure (HF).
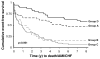
Results: The panel test (IL-6, NT-proBNP, E-selectin) alone detected 60% (95% CI: 49-69; false positive rate: 26%) of subjects that would be classified with myocardial necrosis. Kaplan-Meier and Cox proportional analyses indicated that patients positive by the biomarker panel (including those with cTnI < or =0.04 microg/l) had significantly worse outcomes (death/MI/HF) as compared to those negative by both cTnI and the panel test.
Conclusion: A biomarker panel analyzed early after pain onset can identify individuals at risk for both myocardial necrosis and the combined endpoint of death/MI/HF. Additional prospective studies are required to assess this panel for both early MI detection and to further refine which health outcomes (death, MI, HF) are associated with positive panel results.
Figures

Similar articles
-
Assessment of multiple cardiac biomarkers in non-ST-segment elevation acute coronary syndromes: observations from the MERLIN-TIMI 36 trial.Eur Heart J. 2011 Mar;32(6):697-705. doi: 10.1093/eurheartj/ehq468. Epub 2010 Dec 22. Eur Heart J. 2011. PMID: 21183500 Free PMC article. Clinical Trial.
-
Risk stratification for heart failure and death in an acute coronary syndrome population using inflammatory cytokines and N-terminal pro-brain natriuretic peptide.Clin Chem. 2007 Dec;53(12):2112-8. doi: 10.1373/clinchem.2007.090613. Epub 2007 Oct 11. Clin Chem. 2007. PMID: 17932131
-
Short- and long-term risk stratification using a next-generation, high-sensitivity research cardiac troponin I (hs-cTnI) assay in an emergency department chest pain population.Clin Chem. 2009 Oct;55(10):1809-15. doi: 10.1373/clinchem.2009.127241. Epub 2009 Aug 13. Clin Chem. 2009. PMID: 19679630 Free PMC article.
-
The value of N-terminal proB-type natriuretic peptide for early identification of myocardial infarction in patients with high-risk non-ST-elevation acute coronary syndromes.Clin Chem Lab Med. 2011 Aug;49(8):1359-1365. doi: 10.1515/CCLM.2011.213. Epub 2011 Jun 22. Clin Chem Lab Med. 2011. PMID: 21692686
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
Cited by
-
Blood markers of fibrinolysis and endothelial activation in canine babesiosis.BMC Vet Res. 2017 Mar 31;13(1):82. doi: 10.1186/s12917-017-0995-6. BMC Vet Res. 2017. PMID: 28363279 Free PMC article.
-
Age-related limitations of interleukin-6 in predicting early mortality in acute ST-elevation myocardial infarction.Immun Ageing. 2014 Dec 4;11(1):23. doi: 10.1186/s12979-014-0023-7. eCollection 2014. Immun Ageing. 2014. PMID: 25516764 Free PMC article.
-
Identification of myocardial injury in the emergency setting.Clin Biochem. 2010 Apr;43(6):539-44. doi: 10.1016/j.clinbiochem.2009.12.014. Epub 2009 Dec 21. Clin Biochem. 2010. PMID: 20026097 Free PMC article. Review.
-
Is a pattern of increasing biomarker concentrations important for long-term risk stratification in acute coronary syndrome patients presenting early after the onset of symptoms?Clin Chem. 2008 Apr;54(4):747-51. doi: 10.1373/clinchem.2007.094664. Clin Chem. 2008. PMID: 18375487 Free PMC article.
References
-
- Apple FS, Wu AH, Mair J, et al. Future biomarkers for detection of ischemia and risk stratification in acute coronary syndrome. Clin Chem. 2005;51:810–24. - PubMed
-
- Jaffe AS, Babuin L, Apple FS. Biomarkers in acute cardiac disease: the present and the future. J Am Coll Cardiol. 2006;48:1–11. - PubMed
-
- Jaffe AS. Cardiovascular biomarkers: the state of the art in 2006. Clin Chim Acta. 2007;381:9–13. - PubMed
-
- Lindmark E, Diderholm E, Wallentin L, Siegbahn A. Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease. JAMA. 2001;286:2107–13. - PubMed
-
- Jernberg T, Lindahl B, Siegbahn A, et al. N-Terminal pro-brain natriuretic peptide in relation to inflammation, myocardial necrosis, and the effect of an invasive strategy in unstable coronary artery disease. J Am Coll Cardiol. 2003;42:1909–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

