Myelin oligodendrocyte glycoprotein peptide-induced experimental allergic encephalomyelitis and T cell responses are unaffected by immunoproteasome deficiency
- PMID: 17964666
- PMCID: PMC2175388
- DOI: 10.1016/j.jneuroim.2007.09.024
Myelin oligodendrocyte glycoprotein peptide-induced experimental allergic encephalomyelitis and T cell responses are unaffected by immunoproteasome deficiency
Abstract
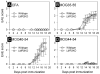
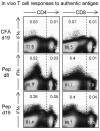
The inoculation of MOG peptides into C57BL/6 mice induces CD4(+) and CD8(+) T cells, and recent work has shown that adoptive transfer of the latter population, after extensive in vitro stimulation, can cause EAE in naïve recipient mice. Herein, we have evaluated the incidence and severity of EAE, and the induction of CD4(+) and CD8(+) T cells, following MOG peptide inoculation of wt mice and of LMP-2KO mice that lack an intact immunoproteasome, a cytoplasmic organelle that is induced by chronic inflammation and that may be important for the presentation of MHC class I epitopes to CD8(+) T cells. We report that EAE, evaluated by both clinical and histological criteria, is similar in LMP-2KO mice and wildtype C57B/6 mice (wt) in response to immunization with MOG peptides MOG(35-55) and MOG(40-54), suggesting that the immunoproteasome does not play a key role in the development of demyelinating disease. Furthermore, and consistent with previous reports, peptide-specific CD8(+) T cells were barely detectable in the CNS of peptide-immunized mice, although peptide-specific CD4(+) T cells were abundant. Therefore, we used a new technique to look for autoreactive CD8(+) T cells in MOG peptide-immunized mice, and we report the identification of CD4(+) and CD8(+) T cells that, as late as 19 days after peptide injection, are actively producing IFNgamma in vivo, in response to in vivo antigen contact.
Figures



References
-
- Abdul-Majid KB, Wefer J, Stadelmann C, Stefferl A, Lassmann H, Olsson T, Harris RA. Comparing the pathogenesis of experimental autoimmune encephalomyelitis in CD4-/- and CD8-/-DBA/1 mice defines qualitative roles of different T cell subsets. J Neuroimmunol. 2003;141:10–19. - PubMed
-
- Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, Hohlfeld R, Friese M, Schroder R, Deckert M, Schmidt S, Ravid R, Rajewsky K. Clonal expansions of CD8+ T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med. 2000;192:393–404. - PMC - PubMed
-
- Barnett LA, Whitton JL, Wada Y, Fujinami RS. Enhancement of autoimmune disease using recombinant vaccinia virus encoding myelin proteolipid protein. J Neuroimmunol. 1993;44:15–25. - PubMed
-
- Barnett LA, Whitton JL, Wang LY, Fujinami RS. Virus encoding an encephalitogenic peptide protects mice from experimental allergic encephalomyelitis. J Neuroimmunol. 1996;64:163–173. - PubMed
-
- Barry MA, Lai WC, Johnston SA. Protection against mycoplasma infection using expression-library immunization. Nature. 1995;377:632–635. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

