Synergistic induction of AHR regulated genes in developmental toxicity from co-exposure to two model PAHs in zebrafish
- PMID: 17964672
- PMCID: PMC2139898
- DOI: 10.1016/j.aquatox.2007.09.005
Synergistic induction of AHR regulated genes in developmental toxicity from co-exposure to two model PAHs in zebrafish
Abstract
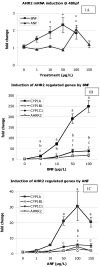
Polycyclic aromatic hydrocarbons (PAHs) are pollutants created by the incomplete combustion of carbon, and are increasing in the environment largely due to the burning of fossil fuels. PAHs occur as complex mixtures, and some combinations have been shown to cause synergistic developmental toxicity in fish embryos, characterized by pericardial edema and craniofacial malformations. Previous studies have indicated that in the zebrafish model, this toxicity is mediated by the aryl hydrocarbon receptor 2 (AHR2), and enhanced by inhibition of CYP1A activity. In this study, we further examined this interaction of the model PAH and AHR agonist beta-naphthoflavone (BNF) with and without the AHR partial agonist/antagonist and CYP1A inhibitor alpha-naphthoflavone (ANF) to determine (1) whether ANF was acting as an AHR antagonist, (2) what alterations BNF and ANF both alone and in combination had on mRNA expression of the AHR regulated genes cytochrome P450 (cyp) 1a, 1 b 1, and 1 c 1, and the AHR repressor (ahrr2) prior to versus during deformity onset, and (3) compare CYP1A enzyme activity with mRNA induction. Zebrafish embryos were exposed from 24-48 or 24-96 hpf to BNF, 1-100 microg/L, ANF, 1-150 microg/L, a BNF+ANF co-exposure (1 microg/L+100 microg/L), or a DMSO solvent control. RNA was extracted and examined by quantitative real-time PCR. Both BNF and ANF each individually resulted in a dose dependent increase CYP1A, CYP1B1, CYP1C1, and AHRR2 mRNA, confirming their activities as AHR agonists. In the BNF+ANF co-exposures prior to deformity onset, expression of these genes was synergistic, and expression levels of the AHR regulated genes resembled the higher doses of BNF alone. Gene induction during deformities was also significantly increased in the co-exposure, but to a lesser magnitude than prior to deformity onset. EROD measurements of CYP1A activity showed ANF inhibited activity induction by BNF in the co-exposure group; this finding is not predicted by mRNA expression, which is synergistically induced in this treatment. This suggests that inhibition of CYP1A activity may alter metabolism and/or increase the half-life of the AHR agonist(s), allowing for increased AHR activation. This study furthers a mechanistic understanding of interactions underlying PAH synergistic toxicity.
Figures



Similar articles
-
The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to zebrafish.Toxicol Sci. 2006 Aug;92(2):526-36. doi: 10.1093/toxsci/kfl011. Epub 2006 May 9. Toxicol Sci. 2006. PMID: 16687390
-
Antioxidant responses and NRF2 in synergistic developmental toxicity of PAHs in zebrafish.Toxicol Sci. 2009 Jun;109(2):217-27. doi: 10.1093/toxsci/kfp038. Epub 2009 Feb 20. Toxicol Sci. 2009. PMID: 19233942 Free PMC article.
-
Zebrafish cardiotoxicity: the effects of CYP1A inhibition and AHR2 knockdown following exposure to weak aryl hydrocarbon receptor agonists.Environ Sci Pollut Res Int. 2015 Jun;22(11):8329-38. doi: 10.1007/s11356-014-3969-2. Epub 2014 Dec 23. Environ Sci Pollut Res Int. 2015. PMID: 25532870 Free PMC article.
-
Mixture effects between different azoles and β-naphthoflavone on the CYP1A biomarker in a fish cell line.Aquat Toxicol. 2015 Jul;164:43-51. doi: 10.1016/j.aquatox.2015.04.016. Epub 2015 Apr 14. Aquat Toxicol. 2015. PMID: 25911577
-
Time-dependent expression and activity of cytochrome P450 1s in early life-stages of the zebrafish (Danio rerio).Environ Sci Pollut Res Int. 2015 Nov;22(21):16319-28. doi: 10.1007/s11356-015-4673-6. Epub 2015 May 22. Environ Sci Pollut Res Int. 2015. PMID: 25994265
Cited by
-
Biological effect monitoring in dab (Limanda limanda) using gene transcript of CYP1A1 or EROD-a comparison.Environ Sci Pollut Res Int. 2008 Oct;15(7):600-5. doi: 10.1007/s11356-008-0048-6. Epub 2008 Oct 14. Environ Sci Pollut Res Int. 2008. PMID: 18853211
-
Fluoranthene, but not benzo[a]pyrene, interacts with hypoxia resulting in pericardial effusion and lordosis in developing zebrafish.Chemosphere. 2008 Dec;74(1):149-54. doi: 10.1016/j.chemosphere.2008.08.016. Epub 2008 Oct 5. Chemosphere. 2008. PMID: 18840388 Free PMC article.
-
Endogenous AhR agonist FICZ accumulates in rainbow trout (Oncorhynchus mykiss) alevins exposed to a mixture of two PAHs, retene and fluoranthene.Ecotoxicology. 2022 Nov;31(9):1382-1389. doi: 10.1007/s10646-022-02593-9. Epub 2022 Oct 11. Ecotoxicology. 2022. PMID: 36219374 Free PMC article.
-
Embryonic Fundulus heteroclitus responses to sediment extracts from differentially contaminated sites in the Elizabeth River, VA.Ecotoxicology. 2019 Nov;28(9):1126-1135. doi: 10.1007/s10646-019-02116-z. Epub 2019 Oct 16. Ecotoxicology. 2019. PMID: 31620948 Free PMC article.
-
Wildlife toxicology: biomarkers of genotoxic exposures at a hazardous waste site.Ecotoxicology. 2009 Oct;18(7):886-98. doi: 10.1007/s10646-009-0350-1. Epub 2009 Jun 17. Ecotoxicology. 2009. PMID: 19533345 Free PMC article.
References
-
- Aluru N, Vuori K, Vijayan MM. Modulation of Ah receptor and CYP1A1 expression by alpha-naphthoflavone in rainbow trout hepatocytes. Comp Biochem Physiol C Toxicol Pharmacol. 2005;141(1):40–49. - PubMed
-
- Andreasen EA, Spitsbergen JM, Tanguay RL, Stegeman JJ, Heideman W, Peterson RE. Tissue-specific expression of AHR2, ARNT2, and CYP1A in zebrafish embryos and larvae: effects of developmental stage and 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure. Toxicol Sci. 2002;68(2):403–419. - PubMed
-
- Billiard SM, Timme-Laragy AR, Wassenberg DM, Cockman C, Di Giulio RT. The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to zebrafish. Toxicol Sci. 2006;92(2):526–536. - PubMed
-
- Brinkworth LC, Hodson PV, Tabash S, Lee P. CYP1A induction and blue sac disease in early developmental stages of rainbow trout (Oncorhynchus mykiss) exposed to retene. J Toxicol Environ Health A. 2003;66(7):627–646. - PubMed
-
- Carney SA, Peterson RE, Heideman W. 2,3,7,8-Tetrachlorodibenzo-p-dioxin activation of the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator pathway causes developmental toxicity through a CYP1A-independent mechanism in zebrafish. Mol Pharmacol. 2004;66(3):512–521. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

