PTHrP increases xenograft growth and promotes integrin alpha6beta4 expression and Akt activation in colon cancer
- PMID: 17964713
- PMCID: PMC2180421
- DOI: 10.1016/j.canlet.2007.09.010
PTHrP increases xenograft growth and promotes integrin alpha6beta4 expression and Akt activation in colon cancer
Abstract
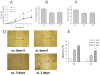
Parathyroid hormone-related protein (PTHrP) is expressed by human colon cancer tissue and cell lines. Expression of PTHrP and phosphatidylinositol 3-kinase (PI3-K) pathway components correlates with the severity of colon carcinoma. Here we observed a positive effect of endogenous PTHrP on LoVo (human colon cancer) cell proliferation, migration, invasion, integrin alpha6 and beta4 expression, and p-Akt levels. There was a direct correlation between PTHrP expression and anchorage-independent cell growth. PTHrP significantly increased xenograft growth; tumors from PTHrP-overexpressing cells showed increased expression of integrins alpha6 and beta4, and PI3-K pathway components. The higher expression of PTHrP in human colon cancer adenocarcinoma vs. normal colonic mucosa was accompanied by increased integrin alpha6 and beta4 levels. Elevated PTHrP expression in colon cancer may thus upregulate integrin alpha6beta4 expression, with consequent PI3-K activation. Targeting PTHrP might result in effective inhibition of tumor growth, migration, and invasion.
Figures
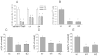




References
-
- Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, CA Cancer. J Clin. 1996;46:5–27. - PubMed
-
- Martin R, Paty P, Fong Y, Grace A, Cohen A, DeMatteo R, Jarnagin W, Blumgart L. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J Am Coll Surg. 2003;197:233–241. - PubMed
-
- Declan Fleming RY. Colorectal cancer screening and follow-up. Surg Oncol. 1998;7:125–137. - PubMed
-
- Kohn EC, Liotta LA. Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res. 1995;55:1856–1862. - PubMed
-
- Goltzman D. Mechanisms of development of osteoblastic metastases. Cancer (Suppl) 1997;80:1581–1587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

