Hypercapnia modulates synaptic interaction of cultured brainstem neurons
- PMID: 17964865
- PMCID: PMC2267290
- DOI: 10.1016/j.resp.2007.09.006
Hypercapnia modulates synaptic interaction of cultured brainstem neurons
Abstract
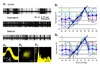
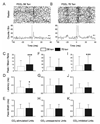
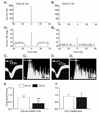
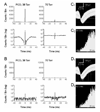
CO(2) is an important metabolic product whose concentrations are constantly monitored by CO(2) chemoreceptors. However, the high systemic CO(2) sensitivity may not be achieved by the CO(2) chemoreceptors without neuronal network processes. To show modulation of network properties during hypercapnia, we studied brainstem neurons dissociated from embryonic rats (P17-19) in multielectrode arrays (MEA) after initial period (3 weeks) of culture. Spike trains of 33,622 pairs of units were analyzed using peri-event histograms (PEH). The amplitude of peri-central peaks between two CO(2)-stimulated units increased and the peak latency decreased during hypercapnia. Similar enhancement of synaptic strength was observed in those sharing a common input. These phenomena were not seen in CO(2)-unresponsive neurons. The amplitude of peri-central peaks between two CO(2) inhibited units also increased without changing latency. Over 60% CO(2)-stimulated neurons studied received mono-/oligosynaptic inputs from other CO(2)-stimulated cells, whereas only approximately 10% CO(2)-unresponsive neurons had such synaptic inputs. A small number of brainstem neurons showed electrical couplings. The coupling efficiency of CO(2)-stimulated but not CO(2)-unresponsive units was suppressed by approximately 50% with high PCO(2). Inhibitory synaptic projections were also found, which was barely affected by hypercapnia. Consistent with the strengthening of excitatory synaptic connections, CO(2) sensitivity of post-synaptic neurons was significantly higher than presynaptic neurons. The difference was eliminated with blockade of presynaptic input. Based on these indirect assessments of synaptic interaction, our PEH analysis suggests that hypercapnia appears to modulate excitatory synaptic transmissions, especially those between CO(2)-stimulated neurons.
Figures




Similar articles
-
High CO2 chemosensitivity versus wide sensing spectrum: a paradoxical problem and its solutions in cultured brainstem neurons.J Physiol. 2007 Feb 1;578(Pt 3):831-41. doi: 10.1113/jphysiol.2006.115758. Epub 2006 Nov 23. J Physiol. 2007. PMID: 17124273 Free PMC article.
-
Galaninergic and hypercapnia-activated neuronal projections to the ventral respiratory column.Brain Struct Funct. 2024 Jun;229(5):1121-1142. doi: 10.1007/s00429-024-02782-8. Epub 2024 Apr 5. Brain Struct Funct. 2024. PMID: 38578351 Free PMC article.
-
Regulation of ventral surface CO2/H+-sensitive neurons by purinergic signalling.J Physiol. 2012 May 1;590(9):2137-50. doi: 10.1113/jphysiol.2012.229666. Epub 2012 Mar 12. J Physiol. 2012. PMID: 22411009 Free PMC article.
-
Role of Astrocytes in Central Respiratory Chemoreception.Adv Exp Med Biol. 2016;949:109-145. doi: 10.1007/978-3-319-40764-7_6. Adv Exp Med Biol. 2016. PMID: 27714687 Review.
-
Central respiratory chemoreception.J Comp Neurol. 2010 Oct 1;518(19):3883-906. doi: 10.1002/cne.22435. J Comp Neurol. 2010. PMID: 20737591 Free PMC article. Review.
Cited by
-
Concomitant differentiation of a population of mouse embryonic stem cells into neuron-like cells and schwann cell-like cells in a slow-flow microfluidic device.Dev Dyn. 2017 Jan;246(1):7-27. doi: 10.1002/dvdy.24466. Epub 2016 Nov 17. Dev Dyn. 2017. PMID: 27761977 Free PMC article.
-
Involvement of TRP channels in the CO₂ chemosensitivity of locus coeruleus neurons.J Neurophysiol. 2011 Jun;105(6):2791-801. doi: 10.1152/jn.00759.2010. Epub 2011 Mar 23. J Neurophysiol. 2011. PMID: 21430274 Free PMC article.
-
Fluoxetine treatment abolishes the in vitro respiratory response to acidosis in neonatal mice.PLoS One. 2010 Oct 26;5(10):e13644. doi: 10.1371/journal.pone.0013644. PLoS One. 2010. PMID: 21048979 Free PMC article.
References
-
- Aertsen AM, Gerstein GL, Habib MK, Palm G. Dynamics of neuronal firing correlation: modulation of "effective connectivity". J Neurophysiol. 1989;61:917. - PubMed
-
- Bou-Flores C, Berger AJ. Gap junctions and inhibitory synapses modulate inspiratory motoneuron synchronization. J Neurophysiol. 2001;85:1543–1551. - PubMed
-
- Buzsaki G. Large-scale recording of neuronal ensembles. Nat.Neurosci. 2004;7:446–451. - PubMed
-
- Christie JM, Bark C, Hormuzdi SG, Helbig I, Monyer H, Westbrook GL. Connexin36 mediates spike synchrony in olfactory bulb glomeruli. Neuron. 2005;46:761–772. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

