Calcineurin promotes hypoxia-inducible factor 1alpha expression by dephosphorylating RACK1 and blocking RACK1 dimerization
- PMID: 17965024
- PMCID: PMC3754800
- DOI: 10.1074/jbc.M705015200
Calcineurin promotes hypoxia-inducible factor 1alpha expression by dephosphorylating RACK1 and blocking RACK1 dimerization
Abstract
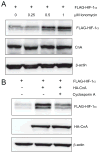
Oxygen homeostasis represents an essential organizing principle of metazoan evolution and biology. Hypoxia-inducible factor 1 (HIF-1) is a master regulator of transcriptional responses to changes in O2 concentration. HIF-1 is a heterodimer of HIF-1alpha and HIF-1beta subunits. O2-dependent degradation of the HIF-1alpha subunit is mediated by prolyl hydroxylase, von Hippel-Lindau protein (VHL)/Elongin-C E3 ubiquitin ligase, and the proteasome. O2-independent degradation of HIF-1alpha is regulated by the competition of RACK1 and HSP90 for binding to HIF-1alpha. RACK1 binding results in the recruitment of the Elongin-C E3 ubiquitin ligase, leading to VHL-independent ubiquitination and degradation of HIF-1alpha. In this report, we show that calcineurin inhibits the ubiquitination and proteasomal degradation of HIF-1alpha. Calcineurin is a serine/threonine phosphatase that is activated by calcium and calmodulin. The phosphatase activity of calcineurin is required for its regulation of HIF-1alpha. RACK1 binds to the catalytic domain of calcineurin and is required for HIF-1alpha degradation induced by the calcineurin inhibitor cyclosporine A. Elongin-C and HIF-1alpha each bind to RACK1 and dimerization of RACK1 is required to recruit Elongin-C to HIF-1alpha. Phosphorylation of RACK1 promotes its dimerization and dephosphorylation by calcineurin inhibits dimerization. Serine 146 within the dimerization domain is phosphorylated and mutation of serine 146 impairs RACK1 dimerization and HIF-1alpha degradation. These results indicate that intracellular calcium levels can regulate HIF-1alpha expression by modulating calcineurin activity and RACK1 dimerization.
Figures






Similar articles
-
RACK1 vs. HSP90: competition for HIF-1 alpha degradation vs. stabilization.Cell Cycle. 2007 Mar 15;6(6):656-9. doi: 10.4161/cc.6.6.3981. Epub 2007 Mar 7. Cell Cycle. 2007. PMID: 17361105 Review.
-
RACK1 competes with HSP90 for binding to HIF-1alpha and is required for O(2)-independent and HSP90 inhibitor-induced degradation of HIF-1alpha.Mol Cell. 2007 Jan 26;25(2):207-17. doi: 10.1016/j.molcel.2007.01.001. Mol Cell. 2007. PMID: 17244529 Free PMC article.
-
Spermidine/spermine N(1)-acetyltransferase-1 binds to hypoxia-inducible factor-1alpha (HIF-1alpha) and RACK1 and promotes ubiquitination and degradation of HIF-1alpha.J Biol Chem. 2007 Nov 16;282(46):33358-33366. doi: 10.1074/jbc.M705627200. Epub 2007 Sep 17. J Biol Chem. 2007. PMID: 17875644
-
Ion channel TRPM8 promotes hypoxic growth of prostate cancer cells via an O2 -independent and RACK1-mediated mechanism of HIF-1α stabilization.J Pathol. 2014 Dec;234(4):514-25. doi: 10.1002/path.4413. Epub 2014 Sep 15. J Pathol. 2014. PMID: 25065497
-
Hypoxia-inducible factor 1 (HIF-1) pathway.Sci STKE. 2007 Oct 9;2007(407):cm8. doi: 10.1126/stke.4072007cm8. Sci STKE. 2007. PMID: 17925579 Review.
Cited by
-
HIF-1α downregulation and apoptosis in hypoxic prostate tumor cells infected with oncolytic mammalian orthoreovirus.Oncotarget. 2014 Jan 30;5(2):561-74. doi: 10.18632/oncotarget.1767. Oncotarget. 2014. PMID: 24504474 Free PMC article.
-
Affinity grid-based cryo-EM of PKC binding to RACK1 on the ribosome.J Struct Biol. 2013 Feb;181(2):190-4. doi: 10.1016/j.jsb.2012.11.006. Epub 2012 Dec 8. J Struct Biol. 2013. PMID: 23228487 Free PMC article.
-
Depletion of the Human Ion Channel TRPM2 in Neuroblastoma Demonstrates Its Key Role in Cell Survival through Modulation of Mitochondrial Reactive Oxygen Species and Bioenergetics.J Biol Chem. 2016 Nov 18;291(47):24449-24464. doi: 10.1074/jbc.M116.747147. Epub 2016 Sep 30. J Biol Chem. 2016. PMID: 27694440 Free PMC article.
-
Hypoxia-inducible factor prolyl hydroxylases as targets for neuroprotection by "antioxidant" metal chelators: From ferroptosis to stroke.Free Radic Biol Med. 2013 Sep;62:26-36. doi: 10.1016/j.freeradbiomed.2013.01.026. Epub 2013 Jan 31. Free Radic Biol Med. 2013. PMID: 23376032 Free PMC article. Review.
-
trans-Endothelial neutrophil migration activates bactericidal function via Piezo1 mechanosensing.Immunity. 2024 Jan 9;57(1):52-67.e10. doi: 10.1016/j.immuni.2023.11.007. Epub 2023 Dec 12. Immunity. 2024. PMID: 38091995 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

