Functional GI disorders: from animal models to drug development
- PMID: 17965064
- PMCID: PMC4130737
- DOI: 10.1136/gut.2006.101675
Functional GI disorders: from animal models to drug development
Abstract
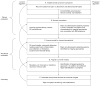
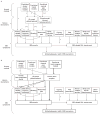
Despite considerable efforts by academic researchers and by the pharmaceutical industry, the development of novel pharmacological treatments for irritable bowel syndrome (IBS) and other functional gastrointestinal (GI) disorders has been slow and disappointing. The traditional approach to identifying and evaluating novel drugs for these symptom-based syndromes has relied on a fairly standard algorithm using animal models, experimental medicine models and clinical trials. In the current article, the empirical basis for this process is reviewed, focusing on the utility of the assessment of visceral hypersensitivity and GI transit, in both animals and humans, as well as the predictive validity of preclinical and clinical models of IBS for identifying successful treatments for IBS symptoms and IBS-related quality of life impairment. A review of published evidence suggests that abdominal pain, defecation-related symptoms (urgency, straining) and psychological factors all contribute to overall symptom severity and to health-related quality of life. Correlations between readouts obtained in preclinical and clinical models and respective symptoms are small, and the ability to predict drug effectiveness for specific as well as for global IBS symptoms is limited. One possible drug development algorithm is proposed which focuses on pharmacological imaging approaches in both preclinical and clinical models, with decreased emphasis on evaluating compounds in symptom-related animal models, and more rapid screening of promising candidate compounds in man.
Conflict of interest statement
Figures

References
-
- Pasricha PJ. Desperately seeking serotonin… A commentary on the withdrawal of tegaserod and the state of drug development for functional and motility disorders. Gastroenterology. 2007;132:2287–90. - PubMed
-
- Mayer EA, Tillisch K, Bradesi S. Review article: Modulation of the brain–gut axis as a therapeutic approach in gastrointestinal disease. Aliment Pharmacol Ther. 2006;24:919–33. - PubMed
-
- Drossman DA, Corazziari E, Delvaux M, et al. ROME III: the functional gastrointestinal disorders. McLean, VA: Degnon Associates; 2006.
-
- Mayer EA, Collins SM. Evolving pathophysiologic models of functional gastrointestinal disorders. Gastroenterology. 2002;122:2032–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
