Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley
- PMID: 17965172
- PMCID: PMC2151677
- DOI: 10.1104/pp.107.110262
Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley
Abstract
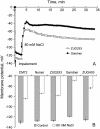
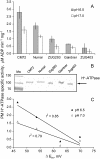
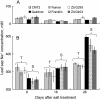
Plant salinity tolerance is a polygenic trait with contributions from genetic, developmental, and physiological interactions, in addition to interactions between the plant and its environment. In this study, we show that in salt-tolerant genotypes of barley (Hordeum vulgare), multiple mechanisms are well combined to withstand saline conditions. These mechanisms include: (1) better control of membrane voltage so retaining a more negative membrane potential; (2) intrinsically higher H(+) pump activity; (3) better ability of root cells to pump Na(+) from the cytosol to the external medium; and (4) higher sensitivity to supplemental Ca(2+). At the same time, no significant difference was found between contrasting cultivars in their unidirectional (22)Na(+) influx or in the density and voltage dependence of depolarization-activated outward-rectifying K(+) channels. Overall, our results are consistent with the idea of the cytosolic K(+)-to-Na(+) ratio being a key determinant of plant salinity tolerance, and suggest multiple pathways of controlling that important feature in salt-tolerant plants.
Figures






References
-
- Apse MP, Aharon GS, Sneddon WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285 1256–1258 - PubMed
-
- Blumwald E (2000) Sodium transport and salt tolerance in plants. Curr Opin Cell Biol 12 431–434 - PubMed
-
- Blumwald E, Aharon GS, Apse MP (2000) Sodium transport in plant cells. Biochim Biophys Acta 1465 140–151 - PubMed
-
- Bordas M, Montesinos C, Dabauza M, Salvador A, Roig LA, Serrano R, Moreno V (1997) Transfer of the yeast salt tolerance gene HAL1 to Cucumis melo L. cultivars and in vitro evaluation of salt tolerance. Transgenic Res 5 1–10 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

