Signature-tagged mutagenesis of Edwardsiella ictaluri identifies virulence-related genes, including a salmonella pathogenicity island 2 class of type III secretion systems
- PMID: 17965213
- PMCID: PMC2168160
- DOI: 10.1128/AEM.01115-07
Signature-tagged mutagenesis of Edwardsiella ictaluri identifies virulence-related genes, including a salmonella pathogenicity island 2 class of type III secretion systems
Abstract
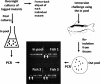
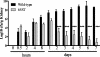
Edwardsiella ictaluri is the leading cause of mortality in channel catfish culture, but little is known about its pathogenesis. The use of signature-tagged mutagenesis in a waterborne infection model resulted in the identification of 50 mutants that were unable to infect/survive in catfish. Nineteen had minitransposon insertions in miscellaneous genes in the chromosome, 10 were in genes that matched to hypothetical proteins, and 13 were in genes that had no significant matches in the NCBI databases. Eight insertions were in genes encoding proteins associated with virulence in other pathogens, including three in genes involved in lipopolysaccharide biosynthesis, three in genes involved in type III secretion systems (TTSS), and two in genes involved in urease activity. With the use of a sequence from a lambda clone carrying several TTSS genes, Blastn analysis of the partially completed E. ictaluri genome identified a 26,135-bp pathogenicity island containing 33 genes of a TTSS with similarity to the Salmonella pathogenicity island 2 class of TTSS. The characterization of a TTSS apparatus mutant indicated that it retained its ability to invade catfish cell lines and macrophages but was defective in intracellular replication. The mutant also invaded catfish tissues in numbers equal to those of invading wild-type E. ictaluri bacteria but replicated poorly and was slowly cleared from the tissues, while the wild type increased in number.
Figures



References
-
- Ainsworth, A. J., G. Capley, P. Waterstreet, and D. Munson. 1986. Use of monoclonal antibodies in the indirect fluorescent antibody technique for the diagnosis of Edwardsiella ictaluri. J. Fish Dis. 9:439-444.
-
- Ainsworth, A. J., and D. X. Chen. 1990. Differences in the phagocytosis of four bacteria by channel catfish neutrophils. Dev. Comp. Immunol. 14:201-209. - PubMed
-
- Aoki, S. K., R. Pamma, A. D. Hernday, J. E. Bickham, B. A. Braaten, and D. A. Low. 2005. Contact-dependent inhibition of growth in Escherichia coli. Science 309:1245-1248. - PubMed
-
- Arnold, D. L., A. Pitman, and R. W. Jackson. 2003. Pathogenicity and other genomic islands in plant pathogenic bacteria. Mol. Plant Pathol. 4:407-420. - PubMed
-
- Ausubel, F. M., R. E. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1994. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, NY.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

