Bone marrow cells adopt the cardiomyogenic fate in vivo
- PMID: 17965233
- PMCID: PMC2077031
- DOI: 10.1073/pnas.0706406104
Bone marrow cells adopt the cardiomyogenic fate in vivo
Abstract
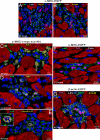
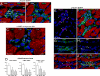
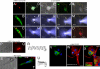
The possibility that adult bone marrow cells (BMCs) retain a remarkable degree of developmental plasticity and acquire the cardiomyocyte lineage after infarction has been challenged, and the notion of BMC transdifferentiation has been questioned. The center of the controversy is the lack of unequivocal evidence in favor of myocardial regeneration by the injection of BMCs in the infarcted heart. Because of the interest in cell-based therapy for heart failure, several approaches including gene reporter assay, genetic tagging, cell genotyping, PCR-based detection of donor genes, and direct immunofluorescence with quantum dots were used to prove or disprove BMC transdifferentiation. Our results indicate that BMCs engraft, survive, and grow within the spared myocardium after infarction by forming junctional complexes with resident myocytes. BMCs and myocytes express at their interface connexin 43 and N-cadherin, and this interaction may be critical for BMCs to adopt the cardiomyogenic fate. With time, a large number of myocytes and coronary vessels are generated. Myocytes show a diploid DNA content and carry, at most, two sex chromosomes. Old and new myocytes show synchronicity in calcium transients, providing strong evidence in favor of the functional coupling of these two cell populations. Thus, BMCs transdifferentiate and acquire the cardiomyogenic and vascular phenotypes restoring the infarcted heart. Together, our studies reveal that locally delivered BMCs generate de novo myocardium composed of integrated cardiomyocytes and coronary vessels. This process occurs independently of cell fusion and ameliorates structurally and functionally the outcome of the heart after infarction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Tosh D, Slack JM. Nat Rev Mol Cell Biol. 2002;3:187–194. - PubMed
-
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, et al. Nature. 2001;410:701–705. - PubMed
-
- Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, et al. Nature. 2004;428:664–668. - PubMed
-
- Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Nature. 2004;428:668–673. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

