Ovarian follicular cells have innate immune capabilities that modulate their endocrine function
- PMID: 17965259
- PMCID: PMC2735812
- DOI: 10.1530/REP-07-0229
Ovarian follicular cells have innate immune capabilities that modulate their endocrine function
Abstract
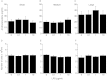
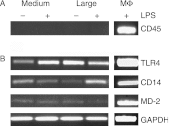
Oestrogens are pivotal in ovarian follicular growth, development and function, with fundamental roles in steroidogenesis, nurturing the oocyte and ovulation. Infections with bacteria such as Escherichia coli cause infertility in mammals at least in part by perturbing ovarian follicle function, characterised by suppression of oestradiol production. Ovarian follicle granulosa cells produce oestradiol by aromatisation of androstenedione from the theca cells, under the regulation of gonadotrophins such as FSH. Many of the effects of E. coli are mediated by its surface molecule lipopolysaccharide (LPS) binding to the Toll-like receptor-4 (TLR4), CD14, MD-2 receptor complex on immune cells, but immune cells are not present inside ovarian follicles. The present study tested the hypothesis that granulosa cells express the TLR4 complex and LPS directly perturbs their secretion of oestradiol. Granulosa cells from recruited or dominant follicles are exposed to LPS in vivo and when they were cultured in the absence of immune cell contamination in vitro they produced less oestradiol when challenged with LPS, although theca cell androstenedione production was unchanged. The suppression of oestradiol production by LPS was associated with down-regulation of transcripts for aromatase in granulosa cells, and did not affect cell survival. Furthermore, these cells expressed TLR4, CD14 and MD-2 transcripts throughout the key stages of follicle growth and development. It appears that granulosa cells have an immune capability to detect bacterial infection, which perturbs follicle steroidogenesis, and this is a likely mechanism by which ovarian follicle growth and function is perturbed during bacterial infection.
Figures



References
-
- Abayasekara DR, Michael AE, Webley GE, Flint AP. Mode of action of prostaglandin F2 alpha in human luteinized granulosa cells: role of protein kinase C. Molecular and Cellular Endocrinology. 1993;97:81–91. - PubMed
-
- Adashi EY, Resnick CE, Croft CS, Payne DW. Tumor necrosis factor alpha inhibits gonadotropin hormonal action in nontransformed ovarian granulosa cells. A modulatory noncytotoxic property. Journal of Biological Chemistry. 1989;264:11591–11597. - PubMed
-
- Akira S, Takeda K. Toll-like receptor signalling. Nature Reviews. Immunology. 2004;4:499–511. - PubMed
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Bao B, Garverick HA, Smith GW, Smith MF, Salfen BE, Youngquist RS. Changes in messenger ribonucleic acid encoding luteinizing hormone receptor, cytochrome P450-side chain cleavage, and aromatase are associated with recruitment and selection of bovine ovarian follicles. Biology of Reproduction. 1997;56:1158–1168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

