Arabidopsis cryptochrome 2 completes its posttranslational life cycle in the nucleus
- PMID: 17965271
- PMCID: PMC2174722
- DOI: 10.1105/tpc.107.053017
Arabidopsis cryptochrome 2 completes its posttranslational life cycle in the nucleus
Abstract
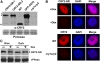
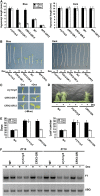
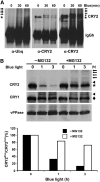
CRY2 is a blue light receptor regulating light inhibition of hypocotyl elongation and photoperiodic flowering in Arabidopsis thaliana. The CRY2 protein is found primarily in the nucleus, and it is known to undergo blue light-dependent phosphorylation and degradation. However, the subcellular location where CRY2 exerts its function or undergoes blue light-dependent phosphorylation and degradation remains unclear. In this study, we analyzed the function and regulation of conditionally nuclear-localized CRY2. Our results show that CRY2 mediates blue light inhibition of hypocotyl elongation and photoperiodic promotion of floral initiation in the nucleus. Consistent with this result and a hypothesis that blue light-dependent phosphorylation is associated with CRY2 function, we demonstrate that CRY2 undergoes blue light-dependent phosphorylation in the nucleus. CRY2 phosphorylation is required for blue light-dependent CRY2 degradation, but only a limited quantity of CRY2 is phosphorylated at any given moment in seedlings exposed to blue light, which explains why continuous blue light illumination is required for CRY2 degradation. Finally, we showed that CRY2 is ubiquitinated in response to blue light and that ubiquitinated CRY2 is degraded by the 26S proteasome in the nucleus. These findings demonstrate that a photoreceptor can complete its posttranslational life cycle (from protein modification, to function, to degradation) inside the nucleus.
Figures



References
-
- Ahmad, M., and Cashmore, A.R. (1993). HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366 162–166. - PubMed
-
- Banerjee, R., Schleicher, E., Meier, S., Viana, R.M., Pokorny, R., Ahmad, M., Bittl, R., and Batschauer, A. (2007). The signaling state of Arabidopsis cryptochrome 2 contains flavin semiquinone. J. Biol. Chem. 282 14916–14922. - PubMed
-
- Bouly, J.P., Giovani, B., Djamei, A., Mueller, M., Zeugner, A., Dudkin, E.A., Batschauer, A., and Ahmad, M. (2003). Novel ATP-binding and autophosphorylation activity associated with Arabidopsis and human cryptochrome-1. Eur. J. Biochem. 270 2921–2928. - PubMed
-
- Bouly, J.P., Schleicher, E., Dionisio-Sese, M., Vandenbussche, F., Van Der Straeten, D., Bakrim, N., Meier, S., Batschauer, A., Galland, P., Bittl, R., and Ahmad, M. (2007). Cryptochrome blue light photoreceptors are activated through interconversion of flavin redox states. J. Biol. Chem. 282 9383–9391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

