The tertiary structure and domain organization of coagulation factor VIII
- PMID: 17965321
- PMCID: PMC2214755
- DOI: 10.1182/blood-2007-08-109918
The tertiary structure and domain organization of coagulation factor VIII
Abstract
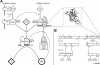
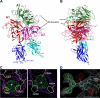
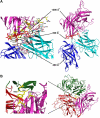
Factor VIII (fVIII) is a serum protein in the coagulation cascade that nucleates the assembly of a membrane-bound protease complex on the surface of activated platelets at the site of a vascular injury. Hemophilia A is caused by a variety of mutations in the factor VIII gene and typically requires replacement therapy with purified protein. We have determined the structure of a fully active, recombinant form of factor VIII (r-fVIII), which consists of a heterodimer of peptides, respectively containing the A1-A2 and A3-C1-C2 domains. The structure permits unambiguous modeling of the relative orientations of the 5 domains of r-fVIII. Comparison of the structures of fVIII, fV, and ceruloplasmin indicates that the location of bound metal ions and of glycosylation, both of which are critical for domain stabilization and association, overlap at some positions but have diverged at others.
Figures

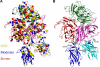
References
-
- Davie EW, Fujikawa K, Kisiel W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. 1991;30:10363–10370. - PubMed
-
- Davie EW, Ratnoff OD. Waterfall sequence for intrinsic blood clotting. Science. 1964;145:1310–1312. - PubMed
-
- Macfarlane RG. An enzyme cascade in the blood clotting mechanism, and its function as a biochemical amplifier. Nature. 1964;202:498–499. - PubMed
-
- Mann KG. Biochemistry and physiology of blood coagulation. Thromb Haemost. 1999;82:165–174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

