Correlation of developmental differences of nuclear transfer embryos cells to the methylation profiles of nuclear transfer donor cells in Swine
- PMID: 17965590
- PMCID: PMC2517257
- DOI: 10.4161/epi.2.3.4844
Correlation of developmental differences of nuclear transfer embryos cells to the methylation profiles of nuclear transfer donor cells in Swine
Abstract
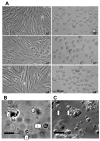
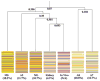
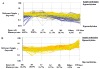
Methylation of DNA is the most commonly studied epigenetic mechanism of developmental competence and somatic cell nuclear transfer (SCNT). Previous studies of epigenetics and the SCNT procedures have examined the effects of different culture media on donor cells and reconstructed embryos, and the methylation status of specific genes in the fetus or live offspring. Here we used a microarray based approach to identify the methylation profiles of SCNT donor cells including three clonal porcine fetal fibroblast-like cell sublines and adult somatic cells selected from kidney and mammary tissues. The methylation profiles of the donor cells were then analyzed with respect to their ability to direct development to the blastocyst stage after nuclear transfer. Clonal cell lines A2, A7 and A8 had blastocyst rates of 11.7%(a), 16.7%(ab) and 20.0%(b), respectively ((ab) p < 0.05). Adult somatic cells included kidney, mammary (large), and mammary (small) also had different blastocyst rates (ab p < 0.05) of 4.2% (a), 10.7% (ab) and 18.3% (b), respectively. For clonal donor cells and for adult somatic cell groups the donor cells with the highest blastocyst rates also had methylation profiles with the lowest similarity to the methylation profiles of the in vivo-produced blastocysts. Conversely, the donor cells with the lowest blastocyst rates had methylation profiles with the highest similarity to the methylation profiles of the in vivo-produced blastocysts. Our findings show there is an inverse correlation to the similarity of the methylation profiles of the donor cells and the in vivo-produced embryos, and to the blastocyst rates following SCNT.
Conflict of interest statement
Potential Conflicts of Interest
The authors are not aware of any conflicts of interest.
Figures
References
-
- Prather RS, Barnes FL, Sims ML, Robl JM, Eyestone WH, First NL. Nuclear transfer in the bovine embryo: assessment of donor nuclei and recipient oocyte. Biology of Reproduction. 1987;37:859–66. - PubMed
-
- Prather RS, Sims MM, First NL. Nuclear transplantation in early pig embryos. Biology of Reproduction. 1989;41:414–8. - PubMed
-
- Willadsen SM. Nuclear transplantation in sheep embryos. Nature. 1986;31:956–62. - PubMed
-
- Humpherys D, Eggan K, Akutsu H, Hochedlinger K, Rideout WM, Biniszkiewicz D, Yanagimachi R, Jaenisch R. Epigenetic instability in ES cells and cloned mice. Science. 2001;293:95–7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
