Effects of chronic in vivo administration of nitroglycerine on ACh-induced endothelium-dependent relaxation in rabbit cerebral arteries
- PMID: 17965730
- PMCID: PMC2199382
- DOI: 10.1038/sj.bjp.0707562
Effects of chronic in vivo administration of nitroglycerine on ACh-induced endothelium-dependent relaxation in rabbit cerebral arteries
Abstract
Background and purpose: In the setting of nitrate tolerance, endothelium-dependent relaxation is reduced in several types of peripheral vessels. However, it is unknown whether chronic in vivo administration of nitroglycerine modulates such relaxation in cerebral arteries.
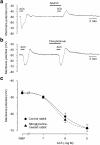
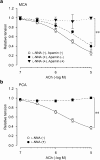
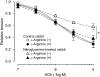
Experimental approach: Isometric force and smooth muscle cell membrane potential were measured in endothelium-intact strips from rabbit middle cerebral artery (MCA) and posterior cerebral artery (PCA).
Key results: ACh (0.1-10 microM) concentration-dependently induced endothelium-dependent relaxation during the contraction induced by histamine in both MCA and PCA. Chronic (10 days) in vivo administration of nitroglycerine reduced the ACh-induced relaxation in PCA but not in MCA, in the presence of the cyclooxygenase inhibitor diclofenac (3 microM). In the presence of the NO-synthase inhibitor N (omega)-nitro-L-arginine (L-NNA, 0.1 mM) plus diclofenac, in MCA from both nitroglycerine-untreated control and -treated rabbits, ACh (0.1-10 microM) induced a smooth muscle cell hyperpolarization and relaxation, and these were blocked by the small-conductance Ca(2+)-activated K(+)-channel inhibitor apamin (0.1 microM), but not by the large- and intermediate-conductance Ca(2+)-activated K(+)-channel inhibitor charybdotoxin (0.1 microM). In contrast, in PCA, ACh (<3 microM) induced neither hyperpolarization nor relaxation under these conditions, suggesting that the endothelium-derived relaxing factor is NO in PCA, whereas endothelium-derived hyperpolarizing factor (EDHF) plays a significant role in MCA.
Conclusions and implications: It is suggested that in rabbit cerebral arteries, the function of the endothelium-derived relaxing factor NO and that of EDHF may be modulated differently by chronic in vivo administration of nitroglycerine.
Figures


Similar articles
-
Role of endothelium in regulation of smooth muscle membrane potential and tone in the rabbit middle cerebral artery.Br J Pharmacol. 1997 Aug;121(7):1315-22. doi: 10.1038/sj.bjp.0701285. Br J Pharmacol. 1997. PMID: 9257909 Free PMC article.
-
Characteristics of attenuated endothelium-dependent relaxation seen in rabbit intrapulmonary vein following chronic nitroglycerine administration.Br J Pharmacol. 2005 May;145(2):193-202. doi: 10.1038/sj.bjp.0706178. Br J Pharmacol. 2005. PMID: 15753949 Free PMC article.
-
Interactions between endothelium-derived relaxing factors in the rat hepatic artery: focus on regulation of EDHF.Br J Pharmacol. 1998 Jul;124(5):992-1000. doi: 10.1038/sj.bjp.0701893. Br J Pharmacol. 1998. PMID: 9692786 Free PMC article.
-
NO/PGI2-independent vasorelaxation and the cytochrome P450 pathway in rabbit carotid artery.Br J Pharmacol. 1997 Feb;120(4):695-701. doi: 10.1038/sj.bjp.0700945. Br J Pharmacol. 1997. PMID: 9051310 Free PMC article.
-
Role of potassium channels in endothelium-dependent relaxation resistant to nitroarginine in the rat hepatic artery.Br J Pharmacol. 1996 Apr;117(7):1600-6. doi: 10.1111/j.1476-5381.1996.tb15327.x. Br J Pharmacol. 1996. PMID: 8730760 Free PMC article.
Cited by
-
Nitroglycerin-induced S-nitrosylation and desensitization of soluble guanylyl cyclase contribute to nitrate tolerance.Circ Res. 2008 Sep 12;103(6):606-14. doi: 10.1161/CIRCRESAHA.108.175133. Epub 2008 Jul 31. Circ Res. 2008. PMID: 18669924 Free PMC article.
-
Characteristics of the actions by which 5-HT affects electrical and mechanical activities in rabbit jugular vein.Br J Pharmacol. 2011 Oct;164(3):979-91. doi: 10.1111/j.1476-5381.2011.01373.x. Br J Pharmacol. 2011. PMID: 21449974 Free PMC article.
-
Endothelium in pharmacology: 30 years on.Br J Pharmacol. 2009 Jun;157(4):491-3. doi: 10.1111/j.1476-5381.2009.00366.x. Br J Pharmacol. 2009. PMID: 19630830 Free PMC article.
-
Characteristic changes in coronary artery at the early hyperglycaemic stage in a rat type 2 diabetes model and the effects of pravastatin.Br J Pharmacol. 2009 Sep;158(2):621-32. doi: 10.1111/j.1476-5381.2009.00348.x. Epub 2009 Jul 23. Br J Pharmacol. 2009. PMID: 19645710 Free PMC article.
References
-
- Berkenboom G, Fontaine D, Unger P, Baldassarre S, Preumont N, Fontaine J. Absence of nitrate tolerance after long-term treatment with ramipril: an endothelium-dependent mechanism. J Cardiovasc Pharmacol. 1999;34:547–553. - PubMed
-
- Busse R, Edwards G, Félétou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. - PubMed
-
- Caramori PRA, Adelman AG, Azevedo ER, Newton GE, Parker AB, Parker JD. Therapy with nitroglycerin increases coronary vasoconstriction in response to acetylcholine. J Am Coll Cardiol. 1998;32:1969–1974. - PubMed
-
- Christiansen I, Iversen HK, Olesen J. Headache characteristics during the development of tolerance to nitrates: pathophysiological implications. Cephalalgia. 2000;20:437–444. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

