Compartmentalization of endocannabinoids into lipid rafts in a dorsal root ganglion cell line
- PMID: 17965731
- PMCID: PMC2219527
- DOI: 10.1038/sj.bjp.0707561
Compartmentalization of endocannabinoids into lipid rafts in a dorsal root ganglion cell line
Abstract
Background and purpose: N-arachidonoyl ethanolamine (AEA) and 2-arachidonoyl glycerol (2-AG) are endogenous cannabinoids binding to the cannabinoid receptors CB1 and CB2 to modulate neuronal excitability and synaptic transmission in primary afferent neurons. To investigate the compartmentalization of the machinery for AEA and 2-AG signalling, we studied their partitioning into lipid raft fractions isolated from a dorsal root ganglion X neuroblastoma cell line (F-11).
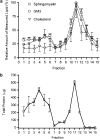
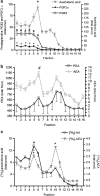
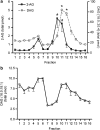
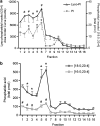
Experimental approach: F-11 cells were homogenized and fractionated using a detergent-free OptiPrep density gradient. All lipids were partially purified from methanolic extracts of the fractions on solid phase cartridges and quantified using liquid chromatography tandem mass spectrometry (LC/MS/MS). Protein distribution was determined by Western blotting.
Key results: Under basal conditions, the endogenous cannabinoid AEA was present in both lipid raft and specific non-lipid raft fractions as was one of its biosynthetic enzymes, NAPE-PLD. The 2-AG precursor 1-stearoyl-2-arachidonoyl-sn-glycerol (DAG), diacylglycerol lipase alpha (DAGLalpha), which cleaves DAG to form 2-AG, and 2-AG were all co-localized with lipid raft markers. CB1 receptors, previously reported to partition into lipid raft fractions, were not detected in F-11 membranes, but CB2 receptors were detected at high levels and partitioned into non-lipid raft fractions.
Conclusions and implications: The biochemical machinery for the production of 2-AG via the putative diacylglycerol pathway is localized within lipid rafts, suggesting that 2-AG synthesis via DAG occurs within these microdomains. The observed co-localization of AEA, 2-AG, and their synthetic enzymes with the reported localization of CB1 raises the possibility of intrinsic-autocrine signalling within lipid raft domains and/or retrograde-paracrine signalling.
Figures

Similar articles
-
Compartmentalization of endocannabinoids into lipid rafts in a microglial cell line devoid of caveolin-1.Br J Pharmacol. 2012 Apr;165(8):2436-49. doi: 10.1111/j.1476-5381.2011.01380.x. Br J Pharmacol. 2012. PMID: 21449981 Free PMC article.
-
Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor.J Neurosci. 2006 May 3;26(18):4740-51. doi: 10.1523/JNEUROSCI.0054-06.2006. J Neurosci. 2006. PMID: 16672646 Free PMC article.
-
Activation of TRPC6 channels promotes endocannabinoid biosynthesis in neuronal CAD cells.Neurochem Int. 2010 Aug;57(1):76-83. doi: 10.1016/j.neuint.2010.05.002. Epub 2010 May 11. Neurochem Int. 2010. PMID: 20466028 Free PMC article.
-
Membrane microdomains and metabolic pathways that define anandamide and 2-arachidonyl glycerol biosynthesis and breakdown.Neuropharmacology. 2008 Dec;55(7):1095-104. doi: 10.1016/j.neuropharm.2008.07.047. Epub 2008 Aug 8. Neuropharmacology. 2008. PMID: 18760289 Free PMC article. Review.
-
New insights into endocannabinoid degradation and its therapeutic potential.Mini Rev Med Chem. 2006 Mar;6(3):257-68. doi: 10.2174/138955706776073466. Mini Rev Med Chem. 2006. PMID: 16515464 Review.
Cited by
-
Membrane environment and endocannabinoid signaling.Front Physiol. 2010 Oct 29;1:140. doi: 10.3389/fphys.2010.00140. eCollection 2010. Front Physiol. 2010. PMID: 21423380 Free PMC article. No abstract available.
-
Programming of neural cells by (endo)cannabinoids: from physiological rules to emerging therapies.Nat Rev Neurosci. 2014 Dec;15(12):786-801. doi: 10.1038/nrn3846. Nat Rev Neurosci. 2014. PMID: 25409697 Free PMC article. Review.
-
Mouse Neuroblastoma CB1 Cannabinoid Receptor-Stimulated [35S]GTPɣS Binding: Total and Antibody-Targeted Gα Protein-Specific Scintillation Proximity Assays.Methods Enzymol. 2017;593:1-21. doi: 10.1016/bs.mie.2017.06.028. Epub 2017 Jul 19. Methods Enzymol. 2017. PMID: 28750799 Free PMC article.
-
Lipid rafts and neurodegeneration: structural and functional roles in physiologic aging and neurodegenerative diseases.J Lipid Res. 2020 May;61(5):636-654. doi: 10.1194/jlr.TR119000427. Epub 2019 Dec 23. J Lipid Res. 2020. PMID: 31871065 Free PMC article. Review.
-
The non-psychoactive plant cannabinoid, cannabidiol affects cholesterol metabolism-related genes in microglial cells.Cell Mol Neurobiol. 2011 Aug;31(6):921-30. doi: 10.1007/s10571-011-9692-3. Epub 2011 Apr 30. Cell Mol Neurobiol. 2011. PMID: 21533611 Free PMC article.
References
-
- Ariga T, Blaine GM, Yoshino H, Dawson G, Kanda T, Zeng GC, et al. Glycosphingolipid composition of murine neuroblastoma cells: O-acetylesterase gene downregulates the expression of O-acetylated GD3. Biochemistry. 1995;34:11500–11507. - PubMed
-
- Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–316. - PubMed
-
- Bari M, Battista N, Fezza F, Finazzi-Agro A, Maccarrone M. Lipid rafts control signaling of type-1 cannabinoid receptors in neuronal cells. Implications for anandamide-induced apoptosis. J Biol Chem. 2005a;280:12212–12220. - PubMed
-
- Bari M, Paradisi A, Pasquariello N, Maccarrone M. Cholesterol-dependent modulation of type 1 cannabinoid receptors in nerve cells. J Neurosci Res. 2005b;81:275–283. - PubMed
-
- Bari M, Spagnuolo P, Fezza F, Oddi S, Pasquariello N, Finazzi-Agro A, et al. Effect of lipid rafts on Cb2 receptor signaling and 2-arachidonoyl-glycerol metabolism in human immune cells. J Immunol. 2006;177:4971–4980. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

