Curcumin inhibits connective tissue growth factor gene expression in activated hepatic stellate cells in vitro by blocking NF-kappaB and ERK signalling
- PMID: 17965732
- PMCID: PMC2241795
- DOI: 10.1038/sj.bjp.0707542
Curcumin inhibits connective tissue growth factor gene expression in activated hepatic stellate cells in vitro by blocking NF-kappaB and ERK signalling
Abstract
Background and purpose: Gene expression of connective tissue growth factor (CTGF) is induced in activated hepatic stellate cells (HSC), the major effectors in hepatic fibrosis, and production of extracellular matrix (ECM) is consequently increased. We previously reported that curcumin, the yellow pigment in curry, suppressed ctgf expression, leading to decreased production of ECM by HSC. The purpose of this study is to evaluate signal transduction pathways involved in the curcumin suppression of ctgf expression in HSC.
Experimental approaches: Transient transfection assays were performed to evaluate effects of activation of signalling pathways on the ctgf promoter activity. Real-time PCR and Western blotting analyses were conducted to determine expression of genes.
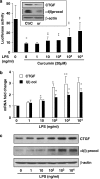
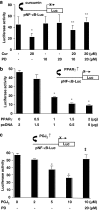
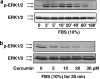
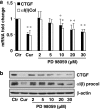
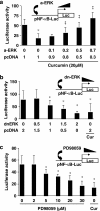
Results: Suppression of ctgf expression by curcumin was dose-dependently reversed by lipopolysaccharide (LPS), an NF-kappaB activator. LPS increased the abundance of CTGF and type I collagen in HSC in vitro. Activation of NF-kappaB by dominant active IkappaB kinase (IKK), or inhibition of NF-kappaB by dominant negative IkappaBalpha, caused the stimulation, or suppression of the ctgf promoter activity, respectively. Curcumin suppressed gene expression of Toll-like receptor-4, leading to the inhibition of NF-kappaB. On the other hand, interruption of ERK signalling by inhibitors or dominant negative ERK, like curcumin, reduced NF-kappaB activity and in ctgf expression. In contrast, the stimulation of ERK signalling by constitutively active ERK prevented the inhibitory effects of curcumin.
Conclusions and implications: These results demonstrate that the interruption of NF-kappaB and ERK signalling by curcumin results in the suppression of ctgf expression in activated HSC in vitro.
Figures
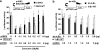
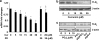
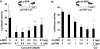
References
-
- Aga M, Watters JJ, Pfeiffer ZA, Wiepz GJ, Sommer JA, Bertics PJ. Evidence for nucleotide receptor modulation of cross talk between MAP kinase and NF-kappa B signaling pathways in murine RAW 264.7 macrophages. Am J Physiol Cell Physiol. 2004;286:C923–C930. - PubMed
-
- Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7. - PubMed
-
- Blom IE, Goldschmeding R, Leask A. Gene regulation of connective tissue growth factor: new targets for antifibrotic therapy. Matrix Biol. 2002;21:473–482. - PubMed
-
- Bourgier C, Haydont V, Milliat F, Francois A, Holler V, Lasser P, et al. Inhibition of Rho kinase modulates radiation induced fibrogenic phenotype in intestinal smooth muscle cells through alteration of the cytoskeleton and connective tissue growth factor expression. Gut. 2005;54:336–343. - PMC - PubMed
-
- Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1488. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

