Role of Ca2+ entry and Ca2+ stores in atypical smooth muscle cell autorhythmicity in the mouse renal pelvis
- PMID: 17965738
- PMCID: PMC2189993
- DOI: 10.1038/sj.bjp.0707535
Role of Ca2+ entry and Ca2+ stores in atypical smooth muscle cell autorhythmicity in the mouse renal pelvis
Abstract
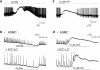
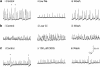
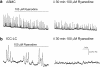
Background and purpose: Electrically active atypical smooth muscle cells (ASMCs) within the renal pelvis have long been considered to act as pacemaker cells driving pelviureteric peristalsis. We have investigated the role of Ca2+ entry and uptake into and release from internal stores in the generation of Ca2+ transients and spontaneous transient depolarizations (STDs) in ASMCs.
Experimental approach: The electrical activity and separately visualized changes in intracellular Ca2+ concentration in typical smooth muscle cells (TSMCs), ASMCs and interstitial cells of Cajal-like cells (ICC-LCs) were recorded using intracellular microelectrodes and a fluorescent Ca2+ indicator, fluo-4.
Results: In 1 microM nifedipine, high frequency (10-30 min(-1)) Ca2+ transients and STDs were recorded in ASMCs, while ICC-LCs displayed low frequency (1-3 min(-1)) Ca2+ transients. All spontaneous electrical activity and Ca2+ transients were blocked upon removal of Ca2+ from the bathing solution, blockade of Ca2+ store uptake with cyclopiazonic acid (CPA) and with 2-aminoethoxy-diphenylborate (2-APB). STD amplitudes were reduced upon removal of the extracellular Na+ or blockade of IP3 dependent Ca2+ store release with neomycin or U73122. Blockade of ryanodine-sensitive Ca2+ release blocked ICC-LC Ca2+ transients but only reduced Ca2+ transient discharge in ASMCs. STDs in ASMCS were also little affected by DIDS, La3+, Gd3+ or by the replacement of extracellular Cl(-) with isethionate.
Conclusions: ASMCs generated Ca2+ transients and cation-selective STDs via mechanisms involving Ca2+ release from IP3-dependent Ca2+ stores, STD stimulation of TSMCs was supported by Ca2+ entry through L type Ca2+ channels and Ca2+ release from ryanodine-sensitive stores.
Figures







Similar articles
-
Spontaneous electrical and Ca2+ signals in typical and atypical smooth muscle cells and interstitial cell of Cajal-like cells of mouse renal pelvis.J Physiol. 2007 Sep 15;583(Pt 3):1049-68. doi: 10.1113/jphysiol.2007.137034. Epub 2007 Jul 26. J Physiol. 2007. PMID: 17656432 Free PMC article.
-
Properties of spontaneous Ca2+ transients recorded from interstitial cells of Cajal-like cells of the rabbit urethra in situ.J Physiol. 2007 Sep 1;583(Pt 2):505-19. doi: 10.1113/jphysiol.2007.136697. Epub 2007 Jul 5. J Physiol. 2007. PMID: 17615099 Free PMC article.
-
Modulators of internal Ca2+ stores and the spontaneous electrical and contractile activity of the guinea-pig renal pelvis.Br J Pharmacol. 2002 Mar;135(6):1363-74. doi: 10.1038/sj.bjp.0704609. Br J Pharmacol. 2002. PMID: 11906949 Free PMC article.
-
Pacemaker Mechanisms Driving Pyeloureteric Peristalsis: Modulatory Role of Interstitial Cells.Adv Exp Med Biol. 2019;1124:77-101. doi: 10.1007/978-981-13-5895-1_3. Adv Exp Med Biol. 2019. PMID: 31183823 Review.
-
Ryanodine receptors in smooth muscle.Front Biosci. 2002 Jul 1;7:d1676-88. doi: 10.2741/a871. Epub 2002 Jul 1. Front Biosci. 2002. PMID: 12086921 Review.
Cited by
-
Potassium and ANO1/ TMEM16A chloride channel profiles distinguish atypical and typical smooth muscle cells from interstitial cells in the mouse renal pelvis.Br J Pharmacol. 2012 Apr;165(7):2389-408. doi: 10.1111/j.1476-5381.2011.01730.x. Br J Pharmacol. 2012. PMID: 22014103 Free PMC article.
-
Calcium signalling in Cajal-like interstitial cells of the lower urinary tract.Nat Rev Urol. 2014 Oct;11(10):555-64. doi: 10.1038/nrurol.2014.241. Epub 2014 Sep 16. Nat Rev Urol. 2014. PMID: 25224445 Review.
-
Isolating and Imaging Live, Intact Pacemaker Regions of Mouse Renal Pelvis by Vibratome Sectioning.J Vis Exp. 2021 Apr 30;(170):10.3791/62040. doi: 10.3791/62040. J Vis Exp. 2021. PMID: 33999021 Free PMC article.
-
Enhanced cellulase production by decreasing intercellular pH through H+-ATPase gene deletion in Trichoderma reesei RUT-C30.Biotechnol Biofuels. 2019 Aug 13;12:195. doi: 10.1186/s13068-019-1536-2. eCollection 2019. Biotechnol Biofuels. 2019. PMID: 31417630 Free PMC article.
-
N,N-dimethylformamide induces cellulase production in the filamentous fungus Trichoderma reesei.Biotechnol Biofuels. 2019 Feb 19;12:36. doi: 10.1186/s13068-019-1375-1. eCollection 2019. Biotechnol Biofuels. 2019. PMID: 30820246 Free PMC article.
References
-
- Aoyama M, Yamada A, Wang J, Ohya S, Furuzono S, Goto T, et al. Requirement of ryanodine receptors for pacemaker Ca2+ activity in ICC and HEK293 cells. J Cell Sc. 2004;117:2813–2825. - PubMed
-
- Boittin FX, Coussin F, Macrez N, Mironneau C, Mironneau J. Inositol 1,4,5-trisphosphate- and ryanodine-sensitive Ca2+ release channel dependent Ca2+ signalling in rat portal vein myocytes. Cell Calcium. 1998;23:303–311. - PubMed
-
- Boittin FX, Macrez N, Halet G, Mironneau J. Norepinephrine-induced Ca2+ waves depend on InsP3 and ryanodine receptor activation in vascular myocytes. Am J Physiol-Cell Physiol. 1999;277:C139–C151. - PubMed
-
- Burdyga T, Wray S. Action potential refractory period in ureter smooth muscle is set by Ca sparks and BK channels. Nature. 2005;436:559–562. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

