Glia-dependent TGF-beta signaling, acting independently of the TH17 pathway, is critical for initiation of murine autoimmune encephalomyelitis
- PMID: 17965773
- PMCID: PMC2040317
- DOI: 10.1172/JCI31763
Glia-dependent TGF-beta signaling, acting independently of the TH17 pathway, is critical for initiation of murine autoimmune encephalomyelitis
Abstract
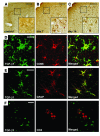
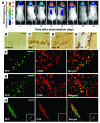
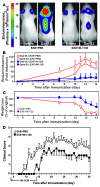
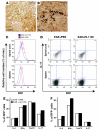
Autoimmune encephalomyelitis, a mouse model for multiple sclerosis, is characterized by the activation of immune cells, demyelination of axons in the CNS, and paralysis. We found that TGF-beta1 synthesis in glial cells and TGF-beta-induced signaling in the CNS were activated several days before the onset of paralysis in mice with autoimmune encephalomyelitis. While early production of TGF-beta1 was observed in glial cells TGF-beta signaling was activated in neurons and later in infiltrating T cells in inflammatory lesions. Systemic treatment with a pharmacological inhibitor of TGF-beta signaling ameliorated the paralytic disease and reduced the accumulation of pathogenic T cells and expression of IL-6 in the CNS. Priming of peripheral T cells was not altered, nor was the generation of TH17 cells, indicating that this effect was directed within the brain, yet affected the immune system. These results suggest that early production of TGF-beta1 in the CNS creates a permissive and dangerous environment for the initiation of autoimmune inflammation, providing a rare example of the brain modulating the immune system. Importantly, inhibition of TGF-beta signaling may have benefits in the treatment of the acute phase of autoimmune CNS inflammation.
Figures



References
-
- Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. - PubMed
-
- Steinman L. Multiple sclerosis: a two-stage disease. Nat. Immunol. 2001;2:762–764. - PubMed
-
- Gold R., Linington C., Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129:1953–1971. - PubMed
-
- Steinman L. Assessment of animal models for MS and demyelinating disease in the design of rational therapy. Neuron. 1999;24:511–514. - PubMed
-
- Massagué J., Blain S.W., Lo R.S. TGF-β signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

