Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients
- PMID: 17965775
- PMCID: PMC2040320
- DOI: 10.1172/JCI32526
Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients
Abstract
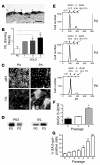
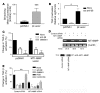
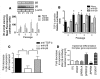
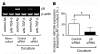
Squamous metaplasia (SM) is common in smokers and is associated with airway obstruction in chronic obstructive pulmonary disease (COPD). A major mechanism of airway obstruction in COPD is thickening of the small airway walls. We asked whether SM actively contributes to airway wall thickening through alteration of epithelial-mesenchymal interactions in COPD. Using immunohistochemical staining, airway morphometry, and fibroblast culture of lung samples from COPD patients; genome-wide analysis of an in vitro model of SM; and in vitro modeling of human airway epithelial-mesenchymal interactions, we provide evidence that SM, through the increased secretion of IL-1beta, induces a fibrotic response in adjacent airway fibroblasts. We identify a pivotal role for integrin-mediated TGF-beta activation in amplifying SM and driving IL-1beta-dependent profibrotic mesenchymal responses. Finally, we show that SM correlates with increased severity of COPD and that fibroblast expression of the integrin alpha(v)beta(8), which is the major mediator of airway fibroblast TGF-beta activation, correlated with disease severity and small airway wall thickening in COPD. Our findings have identified TGF-beta as a potential therapeutic target for COPD.
Figures


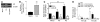
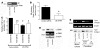


References
-
- Vandevoorde J., et al. Early detection of COPD: A case finding study in general practice. Respir. Med. 2007;101:525–530. - PubMed
-
- International COPD Coalition. 2006. Global burden of COPD summit 2006. http://www.internationalcopd.org/materials/patients/learn/facts.aspx.
-
- Hogg J.C., Macklem P.T., Thurlbeck W.M. Site and nature of airway obstruction in chronic obstructive lung disease. N. Engl. J. Med. 1968;278:1355–1360. - PubMed
-
- Shapiro S.D., Ingenito E.P. The pathogenesis of chronic obstructive pulmonary disease: advances in the past 100 years. Am. J. Respir. Cell Mol. Biol. 2005;32:367–372. - PubMed
-
- Hogg J.C., et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004;350:2645–2653. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AI024674/AI/NIAID NIH HHS/United States
- R37 HL053949/HL/NHLBI NIH HHS/United States
- HL70622/HL/NHLBI NIH HHS/United States
- HL53949/HL/NHLBI NIH HHS/United States
- P30 DK072517/DK/NIDDK NIH HHS/United States
- R01 HL072301/HL/NHLBI NIH HHS/United States
- R01 CA093708/CA/NCI NIH HHS/United States
- HL083950/HL/NHLBI NIH HHS/United States
- AI024674/AI/NIAID NIH HHS/United States
- R01 HL083950/HL/NHLBI NIH HHS/United States
- R01 AI024674/AI/NIAID NIH HHS/United States
- R01 HL085089/HL/NHLBI NIH HHS/United States
- HL63993/HL/NHLBI NIH HHS/United States
- R01 CA095671/CA/NCI NIH HHS/United States
- R01 HL063993/HL/NHLBI NIH HHS/United States
- R01 HL064353/HL/NHLBI NIH HHS/United States
- HL72301/HL/NHLBI NIH HHS/United States
- K02 HL070622/HL/NHLBI NIH HHS/United States
- DK72517/DK/NIDDK NIH HHS/United States
- R01 HL053949/HL/NHLBI NIH HHS/United States
- CA95671/CA/NCI NIH HHS/United States
- HL64353/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

