Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963
- PMID: 17965776
- PMCID: PMC2040321
- DOI: 10.1172/JCI32727
Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963
Abstract
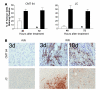
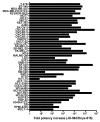
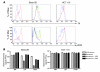
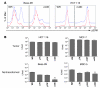
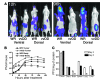
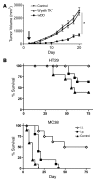
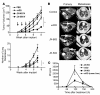
Replication-selective oncolytic viruses (virotherapeutics) are being developed as novel cancer therapies with unique mechanisms of action, but limitations in i.v. delivery to tumors and systemic efficacy have highlighted the need for improved agents for this therapeutic class to realize its potential. Here we describe the rational, stepwise design and evaluation of a systemically effective virotherapeutic (JX-963). We first identified a highly potent poxvirus strain that also trafficked efficiently to human tumors after i.v. administration. This strain was then engineered to target cancer cells with activation of the transcription factor E2F and the EGFR pathway by deletion of the thymidine kinase and vaccinia growth factor genes. For induction of tumor-specific cytotoxic T lymphocytes, we further engineered the virus to express human GM-CSF. JX-963 was more potent than the previously used virotherapeutic Onyx-015 adenovirus and as potent as wild-type vaccinia in all cancer cell lines tested. Significant cancer selectivity of JX-963 was demonstrated in vitro in human tumor cell lines, in vivo in tumor-bearing rabbits, and in primary human surgical samples ex vivo. Intravenous administration led to systemic efficacy against both primary carcinomas and widespread organ-based metastases in immunocompetent mice and rabbits. JX-963 therefore holds promise as a rationally designed, targeted virotherapeutic for the systemic treatment of cancer in humans and warrants clinical testing.
Figures

References
-
- Thorne S.H., Hermiston T., Kirn D. Oncolytic virotherapy: approaches to tumor targeting and enhancing antitumor effects. Semin. Oncol. 2005;32:537–548. - PubMed
-
- Parato K.A., Senger D., Forsyth P.A., Bell J.C. Recent progress in the battle between oncolytic viruses and tumours. Nat. Rev. Cancer. 2005;5:965–976. - PubMed
-
- Smith G.L., Symons J.A., Khanna A., Vanderplasschen A., Alcami A. Vaccinia virus immune evasion. Immunol. Rev. 1997;159:137–154. - PubMed
-
- Hallden G., et al. Novel immunocompetent murine tumor models for the assessment of replication-competent oncolytic adenovirus efficacy. Mol. Ther. 2003;8:412–424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

