Evidence for direct involvement of the capsid protein in HIV infection of nondividing cells
- PMID: 17967060
- PMCID: PMC2042020
- DOI: 10.1371/journal.ppat.0030156
Evidence for direct involvement of the capsid protein in HIV infection of nondividing cells
Abstract
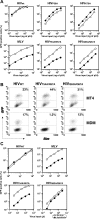
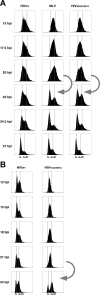
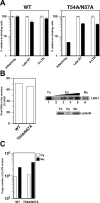
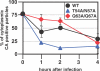
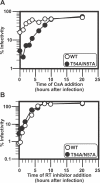
HIV and other lentiviruses can productively infect nondividing cells, whereas most other retroviruses, such as murine leukemia virus, require cell division for efficient infection. However, the determinants for this phenotype have been controversial. Here, we show that HIV-1 capsid (CA) is involved in facilitating HIV infection of nondividing cells because amino acid changes on CA severely disrupt the cell-cycle independence of HIV. One mutant in the N-terminal domain of CA in particular has lost the cell-cycle independence in all cells tested, including primary macrophages. The defect in this mutant appears to be at a stage past nuclear entry. We also find that the loss of cell-cycle independence can be cell-type specific, which suggests that a cellular factor affects the ability of HIV to infect nondividing cells. Our data suggest that CA is directly involved at some step in the viral life cycle that is important for infection of nondividing cells.
Conflict of interest statement
Figures
References
-
- Katz RA, Greger JG, Skalka AM. Effects of cell cycle status on early events in retroviral replication. J Cell Biochem. 2005;94:880–889. - PubMed
-
- Yamashita M, Emerman M. Retroviral infection of non-dividing cells: old and new perspectives. Virology. 2006;344:88–93. - PubMed
-
- Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

