Toll-like receptor 2-mediated interleukin-8 expression in gingival epithelial cells by the Tannerella forsythia leucine-rich repeat protein BspA
- PMID: 17967853
- PMCID: PMC2223669
- DOI: 10.1128/IAI.01139-07
Toll-like receptor 2-mediated interleukin-8 expression in gingival epithelial cells by the Tannerella forsythia leucine-rich repeat protein BspA
Abstract
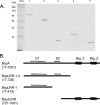
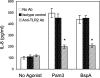
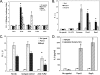
Tannerella forsythia is a gram-negative anaerobe strongly associated with chronic human periodontitis. This bacterium expresses a cell surface-associated and secreted protein, designated BspA, which has been recognized as an important virulence factor. The BspA protein belongs to the leucine-rich repeat (LRR) and bacterial immunoglobulin-like protein families. BspA is, moreover, a multifunctional protein which interacts with a variety of host cells, including monocytes which appear to respond to BspA through Toll-like receptor (TLR) signaling. Since gingival epithelium forms a barrier against periodontal pathogens, this study was undertaken to determine if gingival epithelial cells respond to BspA challenge and if TLRs play any role in BspA recognition. This study was also directed towards identifying the BspA domains responsible for cellular activation. We provide direct evidence for BspA binding to TLR2 and demonstrate that the release of the chemokine interleukin-8 from human gingival epithelial cells by BspA is TLR2 dependent. Furthermore, the LRR domain of BspA is involved in activation of TLR2, while TLR1 serves as a signaling partner. Thus, our findings suggest that BspA is an important modulator of host innate immune responses through activation of TLR2 in cooperation with TLR1.
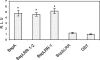
Figures
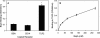
Similar articles
-
Identification of a unique TLR2-interacting peptide motif in a microbial leucine-rich repeat protein.Biochem Biophys Res Commun. 2012 Jul 6;423(3):577-82. doi: 10.1016/j.bbrc.2012.06.008. Epub 2012 Jun 10. Biochem Biophys Res Commun. 2012. PMID: 22695115 Free PMC article.
-
Irsogladine maleate inhibits Porphyromonas gingivalis-mediated expression of toll-like receptor 2 and interleukin-8 in human gingival epithelial cells.J Periodontal Res. 2015 Aug;50(4):486-93. doi: 10.1111/jre.12231. Epub 2014 Sep 20. J Periodontal Res. 2015. PMID: 25244303
-
Farnesol promotes epithelial cell defense against Candida albicans through Toll-like receptor 2 expression, interleukin-6 and human beta-defensin 2 production.Cytokine. 2009 Feb;45(2):132-40. doi: 10.1016/j.cyto.2008.11.011. Epub 2009 Jan 3. Cytokine. 2009. PMID: 19121950
-
Gingival Epithelial Cell Recognition of Lipopolysaccharide.Adv Exp Med Biol. 2019;1197:55-67. doi: 10.1007/978-3-030-28524-1_5. Adv Exp Med Biol. 2019. PMID: 31732934 Review.
-
Association of TLR1, TLR2, TLR4, TLR6, and TIRAP polymorphisms with disease susceptibility.Immunol Res. 2015 Jun;62(2):234-52. doi: 10.1007/s12026-015-8640-6. Immunol Res. 2015. PMID: 25784622 Review.
Cited by
-
The intriguing strategies of Tannerella forsythia's host interaction.Front Oral Health. 2024 May 30;5:1434217. doi: 10.3389/froh.2024.1434217. eCollection 2024. Front Oral Health. 2024. PMID: 38872984 Free PMC article.
-
Levels of serum immunoglobulin G specific to bacterial surface protein A of Tannerella forsythia are related to periodontal status.J Periodontol. 2012 Feb;83(2):228-34. doi: 10.1902/jop.2011.110116. Epub 2011 May 24. J Periodontol. 2012. PMID: 21609257 Free PMC article.
-
An emerging mycoplasma associated with trichomoniasis, vaginal infection and disease.PLoS One. 2014 Oct 22;9(10):e110943. doi: 10.1371/journal.pone.0110943. eCollection 2014. PLoS One. 2014. PMID: 25337710 Free PMC article.
-
DNA from Porphyromonas gingivalis and Tannerella forsythia induce cytokine production in human monocytic cell lines.Mol Oral Microbiol. 2010 Apr;25(2):123-35. doi: 10.1111/j.2041-1014.2009.00551.x. Mol Oral Microbiol. 2010. PMID: 20331800 Free PMC article.
-
Periodontal Pathogens and Their Links to Neuroinflammation and Neurodegeneration.Microorganisms. 2023 Jul 18;11(7):1832. doi: 10.3390/microorganisms11071832. Microorganisms. 2023. PMID: 37513004 Free PMC article. Review.
References
-
- Bulut, Y., E. Faure, L. Thomas, O. Equils, and M. Arditi. 2001. Cooperation of Toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J. Immunol. 167987-994. - PubMed
-
- Duncan, M. J. 2003. Genomics of oral bacteria. Crit. Rev. Oral Biol. Med. 14175-187. - PubMed
-
- Ford, P. J., E. Gemmell, A. Chan, C. L. Carter, P. J. Walker, P. S. Bird, M. J. West, M. P. Cullinan, and G. J. Seymour. 2006. Inflammation, heat shock proteins and periodontal pathogens in atherosclerosis: an immunohistologic study. Oral Microbiol. Immunol. 21206-211. - PubMed
-
- Grabiec, A., G. Meng, S. Fichte, W. Bessler, H. Wagner, and C. J. Kirschning. 2004. Human but not murine Toll-like receptor 2 discriminates between tri-palmitoylated and tri-lauroylated peptides. J. Biol. Chem. 27948004-48012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

