Oral vaccination with Salmonella enterica as a cruzipain-DNA delivery system confers protective immunity against Trypanosoma cruzi
- PMID: 17967857
- PMCID: PMC2223668
- DOI: 10.1128/IAI.01163-07
Oral vaccination with Salmonella enterica as a cruzipain-DNA delivery system confers protective immunity against Trypanosoma cruzi
Abstract
To stimulate both local and systemic immune responses against Trypanosoma cruzi, Salmonella enterica serovar Typhimurium aroA was exploited as a DNA delivery system for cruzipain (SCz). In a murine model we compared SCz alone (GI) or coadministered with Salmonella carrying a plasmid encoding granulocyte-macrophage colony-stimulating factor (GII), as well as protocols in which SCz priming was followed by boosting with recombinant cruzipain (rCz) admixed with either CpG-ODN (GIII) or MALP-2, a synthetic derivative of a macrophage-activating lipopeptide of 2 kDa from Mycoplasma fermentans (GIV). The results showed that protocols that included four oral doses of SCz (GI) elicited mainly a mucosal response characterized by immunoglobulin A (IgA) secretion and proliferation of gut-associated lymphoid tissue cells, with weak systemic responses. In contrast, the protocol that included a boost with rCz plus CpG (GIII) triggered stronger systemic responses in terms of Cz-specific serum IgG titers, splenocyte proliferation, gamma interferon (IFN-gamma) secretion, and delayed-type hypersensitivity response. Trypomastigote challenge of vaccinated mice resulted in significantly lower levels of parasitemia compared to controls. Protection was abolished by depletion of either CD4+ or CD8+ T cells. Parasite control was also evident from the reduction of tissue damage, as revealed by histopathologic studies and serum levels of enzymes that are markers of muscle injury in chronic Chagas' disease (i.e., creatine kinase, aspartate aminotransferase, and lactate dehydrogenase). Enhanced release of IFN-gamma and interleukin-2 was observed in GI and GII upon restimulation of splenocytes in the nonparasitic phase of infection. Our results indicate that Salmonella-mediated delivery of Cz-DNA by itself promotes the elicitation of an immune response that controls T. cruzi infection, thereby reducing parasite loads and subsequent damage to muscle tissues.
Figures
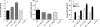
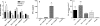
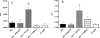

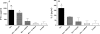
Similar articles
-
Cruzipain and Its Physiological Inhibitor, Chagasin, as a DNA-Based Therapeutic Vaccine Against Trypanosoma cruzi.Front Immunol. 2020 Oct 9;11:565142. doi: 10.3389/fimmu.2020.565142. eCollection 2020. Front Immunol. 2020. PMID: 33162979 Free PMC article.
-
Coadministration of cruzipain and GM-CSF DNAs, a new immunotherapeutic vaccine against Trypanosoma cruzi infection.Hum Vaccin Immunother. 2016;12(2):438-50. doi: 10.1080/21645515.2015.1078044. Hum Vaccin Immunother. 2016. PMID: 26312947 Free PMC article.
-
Prime-boost immunization with cruzipain co-administered with MALP-2 triggers a protective immune response able to decrease parasite burden and tissue injury in an experimental Trypanosoma cruzi infection model.Vaccine. 2008 Apr 7;26(16):1999-2009. doi: 10.1016/j.vaccine.2008.02.011. Epub 2008 Feb 22. Vaccine. 2008. PMID: 18342408
-
Vaccination approaches against Trypanosoma cruzi infection.Expert Rev Vaccines. 2009 Jul;8(7):921-35. doi: 10.1586/erv.09.45. Expert Rev Vaccines. 2009. PMID: 19538117 Review.
-
Immunity and vaccine development efforts against Trypanosoma cruzi.Acta Trop. 2019 Dec;200:105168. doi: 10.1016/j.actatropica.2019.105168. Epub 2019 Sep 9. Acta Trop. 2019. PMID: 31513763 Free PMC article. Review.
Cited by
-
Novel protective antigens expressed by Trypanosoma cruzi amastigotes provide immunity to mice highly susceptible to Chagas' disease.Clin Vaccine Immunol. 2008 Aug;15(8):1292-300. doi: 10.1128/CVI.00142-08. Epub 2008 Jun 25. Clin Vaccine Immunol. 2008. PMID: 18579696 Free PMC article.
-
Recombinant Enolase of Trypanosoma cruzi as a Novel Vaccine Candidate against Chagas Disease in a Mouse Model of Acute Infection.J Immunol Res. 2018 May 7;2018:8964085. doi: 10.1155/2018/8964085. eCollection 2018. J Immunol Res. 2018. PMID: 29854848 Free PMC article.
-
Active and passive immunizations with Bordetella colonization factor A protect mice against respiratory challenge with Bordetella bronchiseptica.Infect Immun. 2009 Feb;77(2):885-95. doi: 10.1128/IAI.01076-08. Epub 2008 Dec 8. Infect Immun. 2009. PMID: 19064638 Free PMC article.
-
Enhancing oral vaccine potency by targeting intestinal M cells.PLoS Pathog. 2010 Nov 11;6(11):e1001147. doi: 10.1371/journal.ppat.1001147. PLoS Pathog. 2010. PMID: 21085599 Free PMC article. Review.
-
Biological and immunological characterization of recombinant Yellow Fever 17D viruses expressing a Trypanosoma cruzi Amastigote Surface Protein-2 CD8+ T cell epitope at two distinct regions of the genome.Virol J. 2011 Mar 18;8:127. doi: 10.1186/1743-422X-8-127. Virol J. 2011. PMID: 21418577 Free PMC article.
References
-
- Aliberti, J. C., J. T. Souto, A. P. Marino, J. Lannes-Vieira, M. M. Teixeira, J. Farber, R. T. Gazzinelli, and J. S. Silva. 2001. Modulation of chemokine production and inflammatory responses in interferon-gamma- and tumor necrosis factor-R1-deficient mice during Trypanosoma cruzi infection. Am. J. Pathol. 581433-1440. - PMC - PubMed
-
- Araujo, A. F., B. C. de Alentar, J. R. Vasconcelos, M. I. Hiyane, C. R. Marinho, M. L. Penido, S. B Boscardin, D. F. Hoft, R. T. Gazzinelli, and M. M. Rodrigues. 2005. CD8+-T-cell-dependent control of Trypanosoma cruzi infection in a highly susceptible mouse strain after immunization with recombinant proteins based on amastigote surface protein 2. Infect. Immun. 736017-6025. - PMC - PubMed
-
- Bellotti, G., E. A. Bocchi, A. V. de Moraes, M. L. Higuchi, M. Barbero-Marcial, E. Sosa, A. Esteves-Filho, R. Kalil, R. Weiss, A. Jatene, and F. Pileggi. 1996. In vivo detection of Trypanosoma cruzi antigens in hearts of patients with chronic Chagas' heart disease. Am. Heart J. 131301-307. - PubMed
-
- Beyer, T., M. Hermann, C. Reiser, W. Bertling, and J. Hess. 2001. Bacterial carriers and virus-like-particles as antigen delivery device: role of dendritic cells in antigen presentation. Curr. Drug Targets Infect. Disord. 1287-302. - PubMed
-
- Bontempi, E., and J. J. Cazzulo. 1990. Digestion of human immunoglobulin G by the major cysteine proteinase (cruzipain) from Trypanosoma cruzi. FEMS Microbiol. Lett. 58337-341. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

