Apoptosis-inducing factor is a target for ubiquitination through interaction with XIAP
- PMID: 17967870
- PMCID: PMC2223279
- DOI: 10.1128/MCB.01065-07
Apoptosis-inducing factor is a target for ubiquitination through interaction with XIAP
Abstract
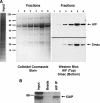
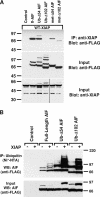
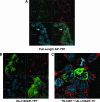
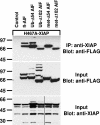
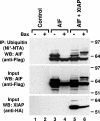
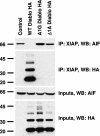
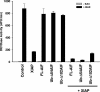
X-linked inhibitor of apoptosis (XIAP) is an inhibitor of apoptotic cell death that protects cells by caspase-dependent and independent mechanisms. In a screen for molecules that participate with XIAP in regulating cellular activities, we identified apoptosis-inducing factor (AIF) as an XIAP binding protein. Baculoviral IAP repeat 2 of XIAP is sufficient for the XIAP/AIF interaction, which is disrupted by Smac/DIABLO. In healthy cells, mature human AIF lacks only the first 54 amino acids, differing significantly from the apoptotic form, which lacks the first 102 amino-terminal residues. Fluorescence complementation and immunoprecipitation experiments revealed that XIAP interacts with both AIF forms. AIF was found to be a target of XIAP-mediated ubiquitination under both normal and apoptotic conditions, and an E3 ubiquitin ligase-deficient XIAP variant displayed a more robust interaction with AIF. Expression of either XIAP or AIF attenuated both basal and antimycin A-stimulated levels of reactive oxygen species (ROS), and when XIAP and AIF were expressed in combination, a cumulative decrease in ROS was observed. These results identify AIF as a new XIAP binding partner and indicate a role for XIAP in regulating cellular ROS.
Figures


Similar articles
-
XIAP as a ubiquitin ligase in cellular signaling.Cell Death Differ. 2010 Jan;17(1):54-60. doi: 10.1038/cdd.2009.81. Cell Death Differ. 2010. PMID: 19590513 Free PMC article. Review.
-
Nondegradative ubiquitination of apoptosis inducing factor (AIF) by X-linked inhibitor of apoptosis at a residue critical for AIF-mediated chromatin degradation.Biochemistry. 2011 Dec 27;50(51):11084-96. doi: 10.1021/bi201483g. Epub 2011 Dec 2. Biochemistry. 2011. PMID: 22103349 Free PMC article.
-
Apoptosis Inducing Factor Binding Protein PGAM5 Triggers Mitophagic Cell Death That Is Inhibited by the Ubiquitin Ligase Activity of X-Linked Inhibitor of Apoptosis.Biochemistry. 2016 Jun 14;55(23):3285-302. doi: 10.1021/acs.biochem.6b00306. Epub 2016 Jun 2. Biochemistry. 2016. PMID: 27218139
-
Smac3, a novel Smac/DIABLO splicing variant, attenuates the stability and apoptosis-inhibiting activity of X-linked inhibitor of apoptosis protein.J Biol Chem. 2003 Dec 26;278(52):52660-72. doi: 10.1074/jbc.M308036200. Epub 2003 Sep 30. J Biol Chem. 2003. PMID: 14523016
-
XIAP: apoptotic brake and promising therapeutic target.Apoptosis. 2001 Aug;6(4):253-61. doi: 10.1023/a:1011379307472. Apoptosis. 2001. PMID: 11445667 Review.
Cited by
-
XIAP as a ubiquitin ligase in cellular signaling.Cell Death Differ. 2010 Jan;17(1):54-60. doi: 10.1038/cdd.2009.81. Cell Death Differ. 2010. PMID: 19590513 Free PMC article. Review.
-
Apoptosis-inducing factor (AIF) at the crossroad of cell survival and cell death: implications for cancer and mitochondrial diseases.Cell Commun Signal. 2025 Jun 4;23(1):264. doi: 10.1186/s12964-025-02272-2. Cell Commun Signal. 2025. PMID: 40468311 Free PMC article. Review.
-
Prediction of localization and interactions of apoptotic proteins.J Biomed Sci. 2009 Jul 6;16(1):59. doi: 10.1186/1423-0127-16-59. J Biomed Sci. 2009. PMID: 19580669 Free PMC article.
-
Phytochemicals: a multitargeted approach to gynecologic cancer therapy.Biomed Res Int. 2014;2014:890141. doi: 10.1155/2014/890141. Epub 2014 Jul 1. Biomed Res Int. 2014. PMID: 25093186 Free PMC article. Review.
-
Tetrathiomolybdate inhibits head and neck cancer metastasis by decreasing tumor cell motility, invasiveness and by promoting tumor cell anoikis.Mol Cancer. 2010 Aug 3;9:206. doi: 10.1186/1476-4598-9-206. Mol Cancer. 2010. Retraction in: Mol Cancer. 2025 Apr 1;24(1):104. doi: 10.1186/s12943-025-02316-8. PMID: 20682068 Free PMC article. Retracted.
References
-
- Arnoult, D., M. Karbowski, and R. J. Youle. 2003. Caspase inhibition prevents the mitochondrial release of apoptosis-inducing factor. Cell Death Differ. 10845-849. - PubMed
-
- Birkey Reffey, S., J. U. Wurthner, W. T. Parks, A. B. Roberts, and C. S. Duckett. 2001. X-linked inhibitor of apoptosis protein functions as a cofactor in transforming growth factor-β signaling. J. Biol. Chem. 27626542-26549. - PubMed
-
- Budihardjo, I., H. Oliver, M. Lutter, X. Luo, and X. Wang. 1999. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 15269-290. - PubMed
-
- Burstein, E., J. E. Hoberg, A. S. Wilkinson, J. M. Rumble, R. A. Csomos, C. M. Komarck, G. N. Maine, J. C. Wilkinson, M. W. Mayo, and C. S. Duckett. 2005. COMMD proteins: a novel family of structural and functional homologs of MURR1. J. Biol. Chem. 28022222-22232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
