Serine 15 phosphorylation of p53 directs its interaction with B56gamma and the tumor suppressor activity of B56gamma-specific protein phosphatase 2A
- PMID: 17967874
- PMCID: PMC2223295
- DOI: 10.1128/MCB.00983-07
Serine 15 phosphorylation of p53 directs its interaction with B56gamma and the tumor suppressor activity of B56gamma-specific protein phosphatase 2A
Abstract
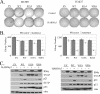
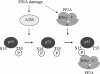
Earlier studies have demonstrated a functional link between B56gamma-specific protein phosphatase 2A (B56gamma-PP2A) and p53 tumor suppressor activity. Upon DNA damage, a complex including B56gamma-PP2A and p53 is formed which leads to Thr55 dephosphorylation of p53, induction of the p53 transcriptional target p21, and the inhibition of cell proliferation. Although an enhanced interaction between p53 and B56gamma is observed after DNA damage, the underlying mechanism and its significance in PP2A tumor-suppressive function remain unclear. In this study, we show that the increased interaction between B56gamma and p53 after DNA damage requires ATM-dependent phosphorylation of p53 at Ser15. In addition, we demonstrate that the B56gamma3-induced inhibition of cell proliferation, induction of cell cycle arrest in G(1), and blockage of anchorage-independent growth are also dependent on Ser15 phosphorylation of p53 and p53-B56gamma interaction. Taken together, our results provide a mechanistic link between Ser15 phosphorylation-mediated p53-B56gamma interaction and the modulation of p53 tumor suppressor activity by PP2A. We also show an important link between ATM activity and the tumor-suppressive function of B56gamma-PP2A.
Figures




Similar articles
-
ATM-mediated phosphorylation activates the tumor-suppressive function of B56γ-PP2A.Oncogene. 2011 Sep 1;30(35):3755-65. doi: 10.1038/onc.2011.95. Epub 2011 Apr 4. Oncogene. 2011. PMID: 21460856
-
A B56gamma mutation in lung cancer disrupts the p53-dependent tumor-suppressor function of protein phosphatase 2A.Oncogene. 2010 Jul 8;29(27):3933-41. doi: 10.1038/onc.2010.161. Epub 2010 May 17. Oncogene. 2010. PMID: 20473327 Free PMC article.
-
B56γ tumor-associated mutations provide new mechanisms for B56γ-PP2A tumor suppressor activity.Mol Cancer Res. 2013 Sep;11(9):995-1003. doi: 10.1158/1541-7786.MCR-12-0633. Epub 2013 May 30. Mol Cancer Res. 2013. PMID: 23723076 Free PMC article.
-
PP2A and tumor radiotherapy.Hereditas. 2020 Aug 26;157(1):36. doi: 10.1186/s41065-020-00149-7. Hereditas. 2020. PMID: 32847617 Free PMC article. Review.
-
All roads lead to PP2A: exploiting the therapeutic potential of this phosphatase.FEBS J. 2016 Mar;283(6):1004-24. doi: 10.1111/febs.13573. Epub 2015 Nov 14. FEBS J. 2016. PMID: 26507691 Free PMC article. Review.
Cited by
-
Prognostic Impact of PPP2R5C Gene Expression in Adult Acute Myeloid Leukemia Patients with Normal Cytogenetics.Indian J Hematol Blood Transfus. 2020 Jan;36(1):37-46. doi: 10.1007/s12288-019-01142-5. Epub 2019 Jun 8. Indian J Hematol Blood Transfus. 2020. PMID: 32158086 Free PMC article.
-
Protein interactomes of protein phosphatase 2A B55 regulatory subunits reveal B55-mediated regulation of replication protein A under replication stress.Sci Rep. 2018 Feb 8;8(1):2683. doi: 10.1038/s41598-018-21040-6. Sci Rep. 2018. PMID: 29422626 Free PMC article.
-
Phosphorylation status of nuclear ribosomal protein S3 is reciprocally regulated by protein kinase C{delta} and protein phosphatase 2A.J Biol Chem. 2009 Aug 7;284(32):21201-8. doi: 10.1074/jbc.M109.018168. Epub 2009 May 20. J Biol Chem. 2009. PMID: 19458393 Free PMC article.
-
Transient PP2A inhibition alleviates normal tissue stem cell susceptibility to cell death during radiotherapy.Cell Death Dis. 2018 May 1;9(5):492. doi: 10.1038/s41419-018-0559-0. Cell Death Dis. 2018. PMID: 29706648 Free PMC article.
-
Pathogenic de novo variants in PPP2R5C cause a neurodevelopmental disorder within the Houge-Janssens syndrome spectrum.Am J Hum Genet. 2025 Mar 6;112(3):554-571. doi: 10.1016/j.ajhg.2025.01.021. Epub 2025 Feb 19. Am J Hum Genet. 2025. PMID: 39978342
References
-
- Bakkenist, C. J., and M. B. Kastan. 2003. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421499-506. - PubMed
-
- Bode, A. M., and Z. Dong. 2004. Post-translational modifications of p53 in tumorigenesis. Nat. Rev. 4793-805. - PubMed
-
- Chen, W., R. Possemato, K. T. Campbell, C. A. Plattner, D. C. Pallas, and W. C. Hahn. 2004. Identification of specific PP2A complexes involved in human cell transformation. Cancer Cell 5127-136. - PubMed
-
- Csortos, C., S. Zolnierowicz, E. Bako, S. D. Durbin, and A. A. DePaoli-Roach. 1996. High complexity in the expression of the B′ subunit of protein phosphatase 2A: evidence for the existence of at least seven novel isoforms. J. Biol. Chem. 2712578-2588. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
