Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by targeted aquaporin-3 gene disruption
- PMID: 17967887
- PMCID: PMC2223314
- DOI: 10.1128/MCB.01482-07
Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by targeted aquaporin-3 gene disruption
Abstract
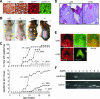
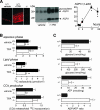
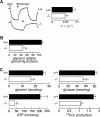
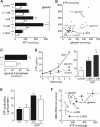
Aquaporin-3 (AQP3) is a water/glycerol-transporting protein expressed strongly at the plasma membranes of basal epidermal cells in skin. We found that human skin squamous cell carcinoma strongly overexpresses AQP3. A novel role for AQP3 in skin tumorigenesis was discovered using mice with targeted AQP3 gene disruption. We found that AQP3-null mice were remarkably resistant to the development of skin tumors following exposure to a tumor initiator and phorbol ester promoter. Though tumor initiator challenge produced comparable apoptotic responses in wild-type and AQP3-null mice, promoter-induced cell proliferation was greatly impaired in the AQP3-null epidermis. Reductions of epidermal cell glycerol, its metabolite glycerol-3-phosphate, and ATP were found in AQP3 deficiency without impairment of mitochondrial function. Glycerol supplementation corrected the reduced proliferation and ATP content in AQP3 deficiency, with cellular glycerol, ATP, and proliferative ability being closely correlated. Our data suggest involvement of AQP3-facilitated glycerol transport in epidermal cell proliferation and tumorigenesis by a novel mechanism implicating cellular glycerol as a key determinant of cellular ATP energy. AQP3 may thus be an important determinant in skin tumorigenesis and hence a novel target for tumor prevention and therapy.
Figures


Similar articles
-
The expression of differentiation markers in aquaporin-3 deficient epidermis.Arch Dermatol Res. 2009 Mar;301(3):245-52. doi: 10.1007/s00403-009-0927-9. Epub 2009 Jan 31. Arch Dermatol Res. 2009. PMID: 19184071 Free PMC article.
-
Roles of aquaporin-3 in the epidermis.J Invest Dermatol. 2008 Sep;128(9):2145-51. doi: 10.1038/jid.2008.70. Epub 2008 Jun 12. J Invest Dermatol. 2008. PMID: 18548108 Review.
-
Aquaporin-3 facilitates epidermal cell migration and proliferation during wound healing.J Mol Med (Berl). 2008 Feb;86(2):221-31. doi: 10.1007/s00109-007-0272-4. Epub 2007 Oct 30. J Mol Med (Berl). 2008. PMID: 17968524
-
Upregulation of aquaporin-3 is involved in keratinocyte proliferation and epidermal hyperplasia.J Invest Dermatol. 2011 Apr;131(4):865-73. doi: 10.1038/jid.2010.395. Epub 2010 Dec 30. J Invest Dermatol. 2011. PMID: 21191421
-
Aquaporin-3 functions as a glycerol transporter in mammalian skin.Biol Cell. 2005 Jul;97(7):479-86. doi: 10.1042/BC20040104. Biol Cell. 2005. PMID: 15966863 Review.
Cited by
-
Effect of intense pulsed light on the expression of aquaporin 3 in rat skin.Lasers Med Sci. 2015 Sep;30(7):1959-65. doi: 10.1007/s10103-015-1788-4. Epub 2015 Aug 1. Lasers Med Sci. 2015. PMID: 26231231
-
The expression of differentiation markers in aquaporin-3 deficient epidermis.Arch Dermatol Res. 2009 Mar;301(3):245-52. doi: 10.1007/s00403-009-0927-9. Epub 2009 Jan 31. Arch Dermatol Res. 2009. PMID: 19184071 Free PMC article.
-
Overexpression of AQP3 Modifies the Cell Cycle and the Proliferation Rate of Mammalian Cells in Culture.PLoS One. 2015 Sep 14;10(9):e0137692. doi: 10.1371/journal.pone.0137692. eCollection 2015. PLoS One. 2015. PMID: 26367709 Free PMC article.
-
Role of aquaporins in cell proliferation: What else beyond water permeability?Channels (Austin). 2016;10(3):185-201. doi: 10.1080/19336950.2016.1139250. Epub 2016 Jan 11. Channels (Austin). 2016. PMID: 26752515 Free PMC article. Review.
-
Signaling Mechanisms and Pharmacological Modulators Governing Diverse Aquaporin Functions in Human Health and Disease.Int J Mol Sci. 2022 Jan 26;23(3):1388. doi: 10.3390/ijms23031388. Int J Mol Sci. 2022. PMID: 35163313 Free PMC article. Review.
References
-
- Baba, H., X. J. Zhang, and R. R. Wolfe. 1995. Glycerol gluconeogenesis in fasting humans. Nutrition 11149-153. - PubMed
-
- Brisson, D., M. C. Vohl, J. St-Pierre, T. J. Hudson, and D. Gaudet. 2001. Glycerol: a neglected variable in metabolic processes? Bioessays 23534-542. - PubMed
-
- DiGiovanni, J. 1992. Multistage carcinogenesis in mouse skin. Pharmacol. Ther. 5463-128. - PubMed
-
- Frigeri, A. M., A. Gropper, F. Umenishi, M. Kawashima, D. Brown, and A. S. Verkman. 1995. Localization of MIWC and GLIP water channel homologs in neuromuscular, epithelial and glandular tissues. J. Cell Sci. 1082993-3002. - PubMed
-
- Fujiyoshi, Y., K. Mitsuoka, B. L. de Groot, A. Philippsen, H. Grubmuller, P. Agre, and A. Engel. 2002. Structure and function of water channels. Curr. Opin. Struct. Biol. 12509-515. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL73856/HL/NHLBI NIH HHS/United States
- R01 EY013574/EY/NEI NIH HHS/United States
- R01 EB000415/EB/NIBIB NIH HHS/United States
- R01 DK035124/DK/NIDDK NIH HHS/United States
- EY13574/EY/NEI NIH HHS/United States
- DK35124/DK/NIDDK NIH HHS/United States
- EB00415/EB/NIBIB NIH HHS/United States
- HL59198/HL/NHLBI NIH HHS/United States
- R01 HL073856/HL/NHLBI NIH HHS/United States
- P30 DK072517/DK/NIDDK NIH HHS/United States
- DK72517/DK/NIDDK NIH HHS/United States
- R01 HL059198/HL/NHLBI NIH HHS/United States
- R37 DK035124/DK/NIDDK NIH HHS/United States
- R37 EB000415/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
