Single and combined silencing of ERK1 and ERK2 reveals their positive contribution to growth signaling depending on their expression levels
- PMID: 17967895
- PMCID: PMC2223286
- DOI: 10.1128/MCB.00800-07
Single and combined silencing of ERK1 and ERK2 reveals their positive contribution to growth signaling depending on their expression levels
Abstract
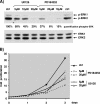
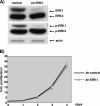
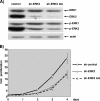
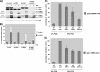
The proteins ERK1 and ERK2 are highly similar, are ubiquitously expressed, and share activators and substrates; however, erk2 gene invalidation is lethal in mice, while erk1 inactivation is not. We ablated ERK1 and/or ERK2 by RNA interference and explored their relative roles in cell proliferation and immediate-early gene (IEG) expression. Reducing expression of either ERK1 or ERK2 lowered IEG induction by serum; however, silencing of only ERK2 slowed down cell proliferation. When both isoforms were silenced simultaneously, compensating activation of the residual pool of ERK1/2 masked a more deleterious effect on cell proliferation. It was only when ERK2 activation was clamped at a limiting level that we demonstrated the positive contribution of ERK1 to cell proliferation. We then established that ERK isoforms are activated indiscriminately and that their expression ratio correlated exactly with their activation ratio. Furthermore, we determined for the first time that ERK1 and ERK2 kinase activities are indistinguishable in vitro and that erk gene dosage is essential for survival of mice. We propose that the expression levels of ERK1 and ERK2 drive their apparent biological differences. Indeed, ERK1 is dispensable in some vertebrates, since it is absent from chicken and frog genomes despite being present in all mammals and fishes sequenced so far.
Figures






Similar articles
-
Total ERK1/2 activity regulates cell proliferation.Cell Cycle. 2009 Mar 1;8(5):705-11. doi: 10.4161/cc.8.5.7734. Epub 2009 Mar 29. Cell Cycle. 2009. PMID: 19242111
-
Genetic demonstration of a redundant role of extracellular signal-regulated kinase 1 (ERK1) and ERK2 mitogen-activated protein kinases in promoting fibroblast proliferation.Mol Cell Biol. 2010 Jun;30(12):2918-32. doi: 10.1128/MCB.00131-10. Epub 2010 Apr 5. Mol Cell Biol. 2010. PMID: 20368360 Free PMC article.
-
Depletion of ERK2 but not ERK1 abrogates oncogenic Ras-induced senescence.Cell Signal. 2013 Dec;25(12):2540-7. doi: 10.1016/j.cellsig.2013.08.014. Epub 2013 Aug 30. Cell Signal. 2013. PMID: 23993963
-
ERK1 and ERK2 mitogen-activated protein kinases affect Ras-dependent cell signaling differentially.J Biol. 2006;5(5):14. doi: 10.1186/jbiol38. J Biol. 2006. PMID: 16805921 Free PMC article.
-
The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition.Oncogene. 2007 May 14;26(22):3227-39. doi: 10.1038/sj.onc.1210414. Oncogene. 2007. PMID: 17496918 Review.
Cited by
-
ERK2 Alone Drives Inflammatory Pain But Cooperates with ERK1 in Sensory Neuron Survival.J Neurosci. 2015 Jun 24;35(25):9491-507. doi: 10.1523/JNEUROSCI.4404-14.2015. J Neurosci. 2015. PMID: 26109671 Free PMC article.
-
Tonic ErbB signaling underlies TGFβ-induced activation of ERK and is required for lens cell epithelial to myofibroblast transition.Mol Biol Cell. 2024 Mar 1;35(3):ar35. doi: 10.1091/mbc.E23-07-0294. Epub 2024 Jan 3. Mol Biol Cell. 2024. PMID: 38170570 Free PMC article.
-
Isoform-Specific Roles of ERK1 and ERK2 in Arteriogenesis.Cells. 2019 Dec 21;9(1):38. doi: 10.3390/cells9010038. Cells. 2019. PMID: 31877781 Free PMC article.
-
miRNA regulation of cytotoxic effects in mouse Sertoli cells exposed to nonylphenol.Reprod Biol Endocrinol. 2011 Sep 14;9:126. doi: 10.1186/1477-7827-9-126. Reprod Biol Endocrinol. 2011. PMID: 21914226 Free PMC article.
-
ERK1 and ERK2 present functional redundancy in tetrapods despite higher evolution rate of ERK1.BMC Evol Biol. 2015 Sep 3;15:179. doi: 10.1186/s12862-015-0450-x. BMC Evol Biol. 2015. PMID: 26336084 Free PMC article.
References
-
- Agrawal, A., S. Dillon, T. L. Denning, and B. Pulendran. 2006. ERK1−/− mice exhibit Th1 cell polarization and increased susceptibility to experimental autoimmune encephalomyelitis. J. Immunol. 1765788-5796. - PubMed
-
- Aury, J. M., O. Jaillon, L. Duret, B. Noel, C. Jubin, B. M. Porcel, B. Segurens, V. Daubin, V. Anthouard, N. Aiach, O. Arnaiz, A. Billaut, J. Beisson, I. Blanc, K. Bouhouche, F. Camara, S. Duharcourt, R. Guigo, D. Gogendeau, M. Katinka, A. M. Keller, R. Kissmehl, C. Klotz, F. Koll, A. Le Mouel, G. Lepere, S. Malinsky, M. Nowacki, J. K. Nowak, H. Plattner, J. Poulain, F. Ruiz, V. Serrano, M. Zagulski, P. Dessen, M. Betermier, J. Weissenbach, C. Scarpelli, V. Schachter, L. Sperling, E. Meyer, J. Cohen, and P. Wincker. 2006. Global trends of whole-genome duplications revealed by the ciliate Paramecium tetraurelia. Nature 444171-178. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
