Hormonal control of the renal immune response and antibacterial host defense by arginine vasopressin
- PMID: 17967904
- PMCID: PMC2118508
- DOI: 10.1084/jem.20071032
Hormonal control of the renal immune response and antibacterial host defense by arginine vasopressin
Abstract
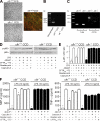
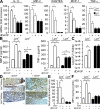
Ascending urinary tract infection (UTI) and pyelonephritis caused by uropathogenic Escherichia coli (UPEC) are very common infections that can cause severe kidney damage. Collecting duct cells, the site of hormonally regulated ion transport and water absorption controlled by vasopressin, are the preferential intrarenal site of bacterial adhesion and initiation of inflammatory response. We investigated the effect of the potent V2 receptor (V2R) agonist deamino-8-D-arginine vasopressin (dDAVP) on the activation of the innate immune response using established and primary cultured collecting duct cells and an experimental model of ascending UTI. dDAVP inhibited Toll-like receptor 4-mediated nuclear factor kappaB activation and chemokine secretion in a V2R-specific manner. The dDAVP-mediated suppression involved activation of protein phosphatase 2A and required an intact cystic fibrosis transmembrane conductance regulator Cl- channel. In vivo infusion of dDAVP induced a marked fall in proinflammatory mediators and neutrophil recruitment, and a dramatic rise in the renal bacterial burden in mice inoculated with UPECs. Conversely, administration of the V2R antagonist SR121463B to UPEC-infected mice stimulated both the local innate response and the antibacterial host defense. These findings evidenced a novel hormonal regulation of innate immune cellular activation and demonstrate that dDAVP is a potent modulator of microbial-induced inflammation in the kidney.
Figures






References
-
- Brown, P., M. Ki, and B. Foxman. 2005. Acute pyelonephritis among adults: cost of illness and considerations for the economic evaluation of therapy. Pharmacoeconomics. 23:1123–1142. - PubMed
-
- Freedman, A.L. 2005. Urologic diseases in North America Project: trends in resource utilization for urinary tract infections in children. J. Urol. 173:949–954. - PubMed
-
- Pelle, G., S. Vimont, P.P. Levy, A. Hertig, N. Ouali, C. Chassin, G. Arlet, A. Rondeau, and A. Vandewalle. 2007. Acute pyelonephritis represents a risk factor impairing long-term kidney graft function. Am. J. Transplant. 7:899–907. - PubMed
-
- Poltorak, A., X. He, I. Smirnova, M.Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, et al. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 282:2085–2088. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

