Comparison of the physicochemical, antifungal, and toxic properties of two liposomal amphotericin B products
- PMID: 17967910
- PMCID: PMC2223902
- DOI: 10.1128/AAC.00870-07
Comparison of the physicochemical, antifungal, and toxic properties of two liposomal amphotericin B products
Abstract
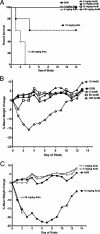
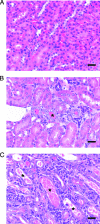
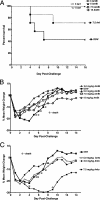
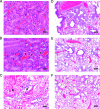
Small unilamellar amphotericin B liposomes can reduce the toxicity of amphotericin B. In this study, we compared the physical, antifungal, pharmocokinetic, and toxic properties of two liposomal amphotericin B products, AmBisome and Anfogen, that have the same chemical composition but are manufactured differently. In vitro tests included determinations of the MICs and the concentrations causing the release of 50% of the intracellular potassium from red blood cells (K50 values) to assess toxicity. The 50% lethal dose (LD50) was evaluated by using uninfected C57BL/6 mice and single intravenous (i.v.) doses of 1 to 100 mg/kg of body weight. Multiple i.v. dosing over 18 days was performed with 0.5, 1.0, or 5.0 mg of Anfogen/kg or 1.0, 5.0, or 25 mg of AmBisome/kg to evaluate chronic toxicity. DBA/2 mice were infected intranasally with 2.5 x 10(6) Aspergillus fumigatus conidia, treated for 3 or 4 days with 3.0, 5.0, or 7.5 mg of Anfogen/kg or 3, 5, 7.5, or 15 mg of AmBisome/kg, and evaluated to assess the toxicity of the drugs to the kidneys (by measurement of blood urea nitrogen and creatinine levels and histopathology) and the drug efficacy. The median particle size was 77.8 nm for AmBisome and 111.5 nm for Anfogen. In vitro K(50) values were significantly lower for Anfogen (0.9 mug/ml) than for AmBisome (20 microg/ml), and the LD50 of AmBisome was >100 mg/kg, versus 10 mg of Anfogen/kg. There was significant renal tubular necrosis in uninfected and infected mice given Anfogen but no tubular necrosis in AmBisome-treated mice. AmBisome at 7.5 or 15 mg/kg was also more efficacious than 7.5 mg of Anfogen/kg for the treatment of pulmonary aspergillosis, based on survival and weight loss data and numbers of CFU per gram of lung. In conclusion, the efficacy and toxicity of these two liposomal amphotericin B products were significantly different, and thus, the products were not comparable.
Figures


Similar articles
-
Toxicity and efficacy differences between liposomal amphotericin B formulations in uninfected and Aspergillus fumigatus infected mice.Med Mycol. 2015 Feb 1;53(2):107-18. doi: 10.1093/mmy/myu070. Epub 2014 Dec 30. Med Mycol. 2015. PMID: 25550388
-
Nanosomal Amphotericin B is an efficacious alternative to Ambisome for fungal therapy.Int J Pharm. 2010 Sep 15;397(1-2):103-8. doi: 10.1016/j.ijpharm.2010.07.003. Epub 2010 Jul 17. Int J Pharm. 2010. PMID: 20621173
-
Assessing the antifungal activity, pharmacokinetics, and tissue distribution of amphotericin B following the administration of Abelcet and AmBisome in combination with caspofungin to rats infected with Aspergillus fumigatus.J Pharm Sci. 2007 Jul;96(7):1737-47. doi: 10.1002/jps.20801. J Pharm Sci. 2007. PMID: 17080414
-
AmBisome: liposomal formulation, structure, mechanism of action and pre-clinical experience.J Antimicrob Chemother. 2002 Feb;49 Suppl 1:21-30. doi: 10.1093/jac/49.suppl_1.21. J Antimicrob Chemother. 2002. PMID: 11801577 Review.
-
Liposomal amphotericin B (AmBisome) for fungal infections in immunocompromised adults and children.Clin Microbiol Infect. 2001;7 Suppl 2:68-79. doi: 10.1111/j.1469-0691.2001.tb00012.x. Clin Microbiol Infect. 2001. PMID: 11525221 Review.
Cited by
-
Self-assembled amphotericin B-loaded polyglutamic acid nanoparticles: preparation, characterization and in vitro potential against Candida albicans.Int J Nanomedicine. 2015 Mar 5;10:1769-90. doi: 10.2147/IJN.S63155. eCollection 2015. Int J Nanomedicine. 2015. PMID: 25784804 Free PMC article.
-
Correlation between Biophysical Properties of Niosomes Elaborated with Chloroquine and Different Tensioactives and Their Transfection Efficiency.Pharmaceutics. 2021 Oct 26;13(11):1787. doi: 10.3390/pharmaceutics13111787. Pharmaceutics. 2021. PMID: 34834203 Free PMC article.
-
Liposomal amphotericin B: a review of its use as empirical therapy in febrile neutropenia and in the treatment of invasive fungal infections.Drugs. 2009;69(3):361-92. doi: 10.2165/00003495-200969030-00010. Drugs. 2009. PMID: 19275278 Review.
-
Differences in efficacy and cytokine profiles following echinocandin or liposomal amphotericin B monotherapy or combination therapy for murine pulmonary or systemic Aspergillus flavus infections.Antimicrob Agents Chemother. 2012 Jan;56(1):218-30. doi: 10.1128/AAC.00607-11. Epub 2011 Oct 3. Antimicrob Agents Chemother. 2012. PMID: 21968353 Free PMC article.
-
NanoDisk containing super aggregated amphotericin B: a high therapeutic index antifungal formulation with enhanced potency.Int J Nanomedicine. 2013;8:4733-43. doi: 10.2147/IJN.S50113. Epub 2013 Dec 12. Int J Nanomedicine. 2013. PMID: 24379661 Free PMC article.
References
-
- Adler-Moore, J. P., and R. T. Proffitt. 1993. Development, characterization, efficacy and mode of action of AmBisome, a unilamellar liposomal formulation of amphotericin B. J. Liposome Res. 3:429-450.
-
- Albert, M. M., L. Stahl-Carroll, M. F. Luther, and J. R. Graybill. 1995. Comparison of liposomal amphotericin B to amphotericin B for treatment of murine cryptococcal meningitis. J. Mycol. Med. 5:1-6.
-
- Allende, M. C., J. W. Lee, P. Francis, K. Garrett, H. Dollenberg, J. Berenguer, C. A. Lyman, P. A. Pizzo, and T. J. Walsh. 1994. Dose-dependent antifungal activity and nephrotoxicity of amphotericin B colloidal dispersion in experimental pulmonary aspergillosis. Antimicrob. Agents Chemother. 38:518-522. - PMC - PubMed
-
- Boswell, G. W., D. Buell, and I. Bekersky. 1998. AmBisome (liposomal amphotericin B): a comparative review. J. Clin. Pharmacol. 38:583-592. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

