Pharmacokinetics and efficacy of piperaquine and chloroquine in Melanesian children with uncomplicated malaria
- PMID: 17967917
- PMCID: PMC2223898
- DOI: 10.1128/AAC.00555-07
Pharmacokinetics and efficacy of piperaquine and chloroquine in Melanesian children with uncomplicated malaria
Abstract
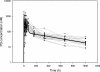
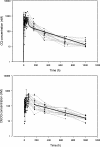
The disposition of chloroquine (CQ) and the related 4-aminoquinoline, piperaquine (PQ), were compared in Papua New Guinean children with uncomplicated malaria. Twenty-two children were randomized to 3 days of PQ phosphate at 20 mg/kg/day (12 mg of PQ base/kg/day) coformulated with dihydroartemisinin (DHA-PQ), and twenty children were randomized to 3 days of CQ at 10 mg base/kg/day with a single dose of sulfadoxine-pyrimethamine (CQ-SP). After a 42-day intensive sampling protocol, PQ, CQ, and its active metabolite monodesethyl-chloroquine (DECQ) were assayed in plasma by using high-performance liquid chromatography. A two-compartment model with first-order absorption was fitted to the PQ and CQ data. There were no significant differences in age, gender, body weight, or admission parasitemia between the two groups. The PCR-corrected 42-day adequate clinical and parasitological responses were 100% for DHA-PQ and 94% for CQ-SP, but P. falciparum reinfections during follow-up were common (33 and 18%, respectively). For PQ, the median volume of distribution at steady state, allowing for bioavailability (Vss/F), was 431 liters/kg (interquartile range [IQR], 283 to 588 liters/kg), the median clearance (CL/F) was 0.85 liters/h/kg (IQR, 0.67 to 1.06 liters/h/kg), the median distribution half-life (t 1/2 alpha) was 0.12 h (IQR, 0.05 to 0.66 h), and the median elimination half-life (t 1/2 beta) was 413 h (IQR, 318 to 516 h). For CQ, the median Vss/F was 154 liters/kg (IQR, 101 to 210 liters/kg), the median CL/F was 0.80 liters/h/kg (IQR, 0.52 to 0.96 liters/h/kg), the median t 1/2 alpha was 0.43 h (IQR, 0.05 to 1.82 h), and the median t 1/2 beta was 233 h (IQR, 206 to 298 h). The noncompartmentally derived median DECQ t 1/2 beta was 290 h (IQR, 236 to 368 h). Combined molar concentrations of DECQ and CQ were higher than those of PQ during the elimination phase. Although PQ has a longer t 1/2 beta than CQ, its prompt distribution and lack of active metabolite may limit its posttreatment malaria-suppressive properties.
Figures
Similar articles
-
Low efficacy of amodiaquine or chloroquine plus sulfadoxine-pyrimethamine against Plasmodium falciparum and P. vivax malaria in Papua New Guinea.Am J Trop Med Hyg. 2007 Nov;77(5):947-54. Am J Trop Med Hyg. 2007. PMID: 17984359 Clinical Trial.
-
Population pharmacokinetics, tolerability, and safety of dihydroartemisinin-piperaquine and sulfadoxine-pyrimethamine-piperaquine in pregnant and nonpregnant Papua New Guinean women.Antimicrob Agents Chemother. 2015 Jul;59(7):4260-71. doi: 10.1128/AAC.00326-15. Epub 2015 May 11. Antimicrob Agents Chemother. 2015. PMID: 25963981 Free PMC article.
-
Therapeutic efficacies of artesunate-sulfadoxine-pyrimethamine and chloroquine-sulfadoxine-pyrimethamine in vivax malaria pilot studies: relationship to Plasmodium vivax dhfr mutations.Antimicrob Agents Chemother. 2002 Dec;46(12):3947-53. doi: 10.1128/AAC.46.12.3947-3953.2002. Antimicrob Agents Chemother. 2002. PMID: 12435700 Free PMC article. Clinical Trial.
-
Metabolic disruptions in P. vivax malaria: Insights from four antimalarial treatment regimens.Acta Trop. 2025 Aug;268:107722. doi: 10.1016/j.actatropica.2025.107722. Epub 2025 Jul 2. Acta Trop. 2025. PMID: 40615061 Review.
-
Efficacy and safety of dihydroartemisinin-piperaquine for treatment of Plasmodium vivax malaria in endemic countries: meta-analysis of randomized controlled studies.PLoS One. 2013 Dec 3;8(12):e78819. doi: 10.1371/journal.pone.0078819. eCollection 2013. PLoS One. 2013. PMID: 24312446 Free PMC article. Review.
Cited by
-
Effect of Dihydroartemisinin-Piperaquine on the Pharmacokinetics of Praziquantel for Treatment of Schistosoma mansoni Infection.Pharmaceuticals (Basel). 2021 Apr 23;14(5):400. doi: 10.3390/ph14050400. Pharmaceuticals (Basel). 2021. PMID: 33922522 Free PMC article.
-
Pharmacokinetics of chloroquine and monodesethylchloroquine in pregnancy.Antimicrob Agents Chemother. 2010 Mar;54(3):1186-92. doi: 10.1128/AAC.01269-09. Epub 2010 Jan 19. Antimicrob Agents Chemother. 2010. PMID: 20086162 Free PMC article. Clinical Trial.
-
Model-Informed Repurposing of Medicines for SARS-CoV-2: Extrapolation of Antiviral Activity and Dose Rationale for Paediatric Patients.Pharmaceutics. 2021 Aug 19;13(8):1299. doi: 10.3390/pharmaceutics13081299. Pharmaceutics. 2021. PMID: 34452260 Free PMC article.
-
Artemisinin-naphthoquine versus artemether-lumefantrine for uncomplicated malaria in Papua New Guinean children: an open-label randomized trial.PLoS Med. 2014 Dec 30;11(12):e1001773. doi: 10.1371/journal.pmed.1001773. eCollection 2014 Dec. PLoS Med. 2014. PMID: 25549086 Free PMC article. Clinical Trial.
-
Transfer of chloroquine and desethylchloroquine across the placenta and into milk in Melanesian mothers.Br J Clin Pharmacol. 2008 May;65(5):674-9. doi: 10.1111/j.1365-2125.2008.03111.x. Epub 2008 Feb 15. Br J Clin Pharmacol. 2008. PMID: 18279478 Free PMC article.
References
-
- Adjepon-Yamoah, K. K., D. Ofori-Adjei, N. M. Woolhouse, and B. Lindstrom. 1986. Whole-blood single-dose kinetics of chloroquine and desethylchloroquine in Africans. Ther. Drug Monit. 8:195-199. - PubMed
-
- Anonymous. 2000. Standard treatment of common illnesses of children in Papua New Guinea, 7th ed. Papua New Guinea Department of Health, Port Moresby, Papua New Guinea.
-
- Bustos, D. G., J. E. Lazaro, F. Gay, A. Pottier, C. J. Laracas, B. Traore, and B. Diquet. 2002. Pharmacokinetics of sequential and simultaneous treatment with the combination chloroquine and sulfadoxine-pyrimethamine in acute uncomplicated Plasmodium falciparum malaria in the Philippines. Trop. Med. Int. Health 7:584-591. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

