Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells
- PMID: 17967941
- PMCID: PMC2200823
- DOI: 10.1182/blood-2007-06-094482
Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells
Abstract
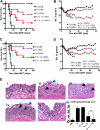
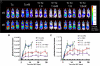
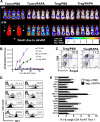
Based on their ability to control T-cell homeostasis, Foxp3(+)CD4(+)CD25(+) regulatory T cells (Tregs) are being considered for treatment of autoimmune disorders and acute graft-versus-host disease (aGVHD). When combining Tregs with the immunosuppressant rapamycin (RAPA), we observed reduced alloreactive conventional T-cell (Tconv) expansion and aGVHD lethality compared with each treatment alone. This synergistic in vivo protection was paralleled by intact expansion of polyclonal Tregs with conserved high FoxP3 expression. In contrast to Tconv, activation of Tregs with alloantigen and interleukin-2 preferentially led to signal transducer and activator of transcription 5 (STAT5) phosphorylation and not phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway activity. Expression of phosphatase and tensin homolog deleted on chromosome 10 (PTEN), a negative regulator of the PI3K/Akt/mTOR pathway, remained high in Tregs but not Tconv during stimulation. Conversely, targeted deletion of PTEN increased susceptibility of Tregs to mTOR inhibition by RAPA. Differential impact of RAPA as a result of reduced usage of the mTOR pathway in Tregs compared with conventional T cells explains the synergistic effect of RAPA and Tregs in aGVHD protection, which has important implications for clinical trials using Tregs.
Figures


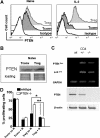
 ) or PTEN-deficient (
) or PTEN-deficient ( ) Tconv or Treg after 48-hour stimulation with (CD11c+ H-2kd, 30 Gy γ-irradiation) and IL-2 (100 IU/mL), in the presence or absence of RAPA (R, 10 ng/mL) as indicated (*P < .05; **P < .01). Proliferation was assessed by serial CFSE dilution.
) Tconv or Treg after 48-hour stimulation with (CD11c+ H-2kd, 30 Gy γ-irradiation) and IL-2 (100 IU/mL), in the presence or absence of RAPA (R, 10 ng/mL) as indicated (*P < .05; **P < .01). Proliferation was assessed by serial CFSE dilution.
References
-
- Sakaguchi S. Naturally arising CD4(+) regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. - PubMed
-
- Wu Y, Borde M, Heissmeyer V, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. - PubMed
-
- Ho L, Crabtree G. A Foxy tango with NFAT. Nat Immunol. 2006;7:906–908. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

