Viable but nonculturable Vibrio cholerae O1 in biofilms in the aquatic environment and their role in cholera transmission
- PMID: 17968017
- PMCID: PMC2077051
- DOI: 10.1073/pnas.0705599104
Viable but nonculturable Vibrio cholerae O1 in biofilms in the aquatic environment and their role in cholera transmission
Abstract
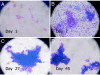
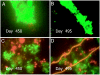
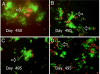
Vibrio cholerae persists in aquatic environments predominantly in a nonculturable state. In this study coccoid, nonculturable V. cholerae O1 in biofilms maintained for 495 days in Mathbaria, Bangladesh, pond water became culturable upon animal passage. Culturability, biofilm formation, and the wbe, ctxA, and rstR2 genes were monitored by culture, direct fluorescent antibody (DFA), and multiplex PCR. DFA counts were not possible after formation of biofilm. Furthermore, wbe, but not ctxA, were amplifiable, even after incubation for 54 and 68 days at room temperature ( approximately 25 degrees C) and 4 degrees C, respectively, when no growth was detectable. Slower biofilm formation and extended culturability were observed for cultures incubated at 4 degrees C, compared with approximately 25 degrees C, suggesting biofilm production to be temperature dependent and linked to loss of culturability. Small colonies appearing after incubation in microcosms for 54 and 68 days at 25 degrees C and 4 degrees C, respectively, were wbe positive and ctxA and rstR2 negative, indicating loss of bacteriophage CTXphi. The coccoid V. cholerae O1 observed as free cells in microcosms incubated for 495 days could not be cultured, but biofilms in the same microcosms yielded culturable cells. It is concluded that biofilms can act as a reservoir for V. cholerae O1 between epidemics because of its long-term viability in biofilms. In contrast to biofilms produced in Mathbaria pond water, V. cholerae O1 in biofilms present in cholera stools and incubated under identical conditions as the Mathbaria pond water biofilms could not be cultured after 2 months, indicating that those V. cholerae cells freshly discharged into the environment are significantly less robust than cells adapted to environmental conditions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Sack RB, Siddique AK, Longini IM, Nizam A, Yunus M, Islam MS, Morris JG, Ali A, Huq A, Nair GB, et al. J Infect Dis. 2003;187:96–101. - PubMed
-
- Colwell RR, Spira WM. In: The Ecology of Vibrio cholerae. Barua D, Greenough WBI, editors. New York: Plenum; 1992. pp. 107–127.
-
- Xu HS, Roberts N, Singleton FL, Atwell RW, Grimes DJ, Colwell RR. Microb Ecol. 1982;8:313–323. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

