Aquaporins and cell migration
- PMID: 17968585
- PMCID: PMC3595095
- DOI: 10.1007/s00424-007-0357-5
Aquaporins and cell migration
Abstract
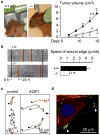
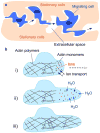
Aquaporin (AQP) water channels are expressed primarily in cell plasma membranes. In this paper, we review recent evidence that AQPs facilitate cell migration. AQP-dependent cell migration has been found in a variety of cell types in vitro and in mice in vivo. AQP1 deletion reduces endothelial cell migration, limiting tumor angiogenesis and growth. AQP4 deletion slows the migration of reactive astrocytes, impairing glial scarring after brain stab injury. AQP1-expressing tumor cells have enhanced metastatic potential and local infiltration. Impaired cell migration has also been seen in AQP1-deficient proximal tubule epithelial cells, and AQP3-deficient corneal epithelial cells, enterocytes, and skin keratinocytes. The mechanisms by which AQPs enhance cell migration are under investigation. We propose that, as a consequence of actin polymerization/depolymerization and transmembrane ionic fluxes, the cytoplasm adjacent to the leading edge of migrating cells undergoes rapid changes in osmolality. AQPs could thus facilitate osmotic water flow across the plasma membrane in cell protrusions that form during migration. AQP-dependent cell migration has potentially broad implications in angiogenesis, tumor metastasis, wound healing, glial scarring, and other events requiring rapid, directed cell movement. AQP inhibitors may thus have therapeutic potential in modulating these events, such as slowing tumor growth and spread, and reducing glial scarring after injury to allow neuronal regeneration.
Figures


Similar articles
-
Increased migration and metastatic potential of tumor cells expressing aquaporin water channels.FASEB J. 2006 Sep;20(11):1892-4. doi: 10.1096/fj.06-5930fje. Epub 2006 Jul 3. FASEB J. 2006. PMID: 16818469
-
Aquaporins--new players in cancer biology.J Mol Med (Berl). 2008 May;86(5):523-9. doi: 10.1007/s00109-008-0303-9. Epub 2008 Mar 1. J Mol Med (Berl). 2008. PMID: 18311471 Free PMC article. Review.
-
Expression and function of water channels (aquaporins) in migrating malignant astrocytes.Glia. 2007 Aug 1;55(10):1034-43. doi: 10.1002/glia.20524. Glia. 2007. PMID: 17549682 Free PMC article.
-
Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption.Nature. 2005 Apr 7;434(7034):786-92. doi: 10.1038/nature03460. Nature. 2005. PMID: 15815633
-
Role of aquaporins in corneal healing post chemical injury.Exp Eye Res. 2023 Mar;228:109390. doi: 10.1016/j.exer.2023.109390. Epub 2023 Jan 22. Exp Eye Res. 2023. PMID: 36696947 Free PMC article. Review.
Cited by
-
Predicting the effects of radiotherapy based on diffusion kurtosis imaging in a xenograft mouse model of esophageal carcinoma.Exp Ther Med. 2021 Apr;21(4):327. doi: 10.3892/etm.2021.9758. Epub 2021 Feb 5. Exp Ther Med. 2021. PMID: 33732300 Free PMC article.
-
Buffer-dependent regulation of aquaporin-1 expression and function in human peritoneal mesothelial cells.Pediatr Nephrol. 2012 Jul;27(7):1165-77. doi: 10.1007/s00467-012-2120-1. Epub 2012 Mar 1. Pediatr Nephrol. 2012. PMID: 22382466
-
Cellular Distribution of Aquaporin 3, 7 and 9 in the Male Reproductive System: A Lesson from Bovine Study (Bos taurus).Int J Mol Sci. 2024 Jan 26;25(3):1567. doi: 10.3390/ijms25031567. Int J Mol Sci. 2024. PMID: 38338845 Free PMC article.
-
Inhibition of AQP1 Hampers Osteosarcoma and Hepatocellular Carcinoma Progression Mediated by Bone Marrow-Derived Mesenchymal Stem Cells.Int J Mol Sci. 2016 Jul 11;17(7):1102. doi: 10.3390/ijms17071102. Int J Mol Sci. 2016. PMID: 27409610 Free PMC article.
-
Durotaxis Index of 3T3 Fibroblast Cells Scales with Stiff-to-Soft Membrane Tension Polarity.Biophys J. 2020 Oct 6;119(7):1427-1438. doi: 10.1016/j.bpj.2020.07.039. Epub 2020 Aug 20. Biophys J. 2020. PMID: 32898477 Free PMC article.
References
-
- Auguste KI, Jin S, Uchida K, Yan D, Manley GT, Papadopoulos MC, Verkman AS. Greatly impaired migration of implanted aquaporin-4-deficient astroglial cells in mouse brain toward a site of injury. FASEB J. 2007;21:108–116. - PubMed
-
- Barber MA, Welch HC. PI3K and RAC signalling in leukocyte and cancer cell migration. Bull Cancer. 2006;93:E44–E52. - PubMed
-
- Crane J, Verkman AS. Long-range non-anomalous diffusion of quantum dot-labeled aquaporin-1 water channels in the cell plasma membrane. Biophys J. 2007 http://dx.doi.org/10.1529/biophysj.107.115121 (in press) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL73856/HL/NHLBI NIH HHS/United States
- R01 EY013574/EY/NEI NIH HHS/United States
- R01 EB000415/EB/NIBIB NIH HHS/United States
- R01 DK035124/DK/NIDDK NIH HHS/United States
- EY13574/EY/NEI NIH HHS/United States
- DK35124/DK/NIDDK NIH HHS/United States
- EB00415/EB/NIBIB NIH HHS/United States
- HL59198/HL/NHLBI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R01 HL073856/HL/NHLBI NIH HHS/United States
- P30 DK072517/DK/NIDDK NIH HHS/United States
- DK72517/DK/NIDDK NIH HHS/United States
- R01 HL059198/HL/NHLBI NIH HHS/United States
- R37 DK035124/DK/NIDDK NIH HHS/United States
- R37 EB000415/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

