Deficiency of the type I interferon receptor protects mice from experimental lupus
- PMID: 17968932
- PMCID: PMC2909118
- DOI: 10.1002/art.23023
Deficiency of the type I interferon receptor protects mice from experimental lupus
Abstract
Objective: Systemic lupus erythematosus (SLE) is diagnosed according to a spectrum of clinical manifestations and autoantibodies associated with abnormal expression of type I interferon (IFN-I)-stimulated genes (ISGs). The role of IFN-I in the pathogenesis of SLE remains uncertain, partly due to the lack of suitable animal models. The objective of this study was to examine the role of IFN-I signaling in the pathogenesis of murine lupus induced by 2,6,10,14-tetramethylpentadecane (TMPD).
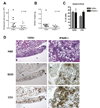
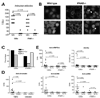
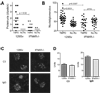
Methods: IFN-I receptor-deficient (IFNAR(-/-)) 129Sv mice and wild-type (WT) 129Sv control mice were treated intraperitoneally with TMPD. The expression of ISGs was measured by real-time polymerase chain reaction. Autoantibody production was evaluated by immunofluorescence and enzyme-linked immunosorbent assay. Proteinuria and renal glomerular cellularity were measured and renal immune complexes were examined by immunofluorescence.
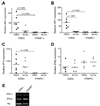
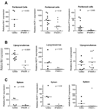
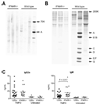
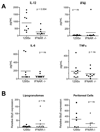
Results: Increased ISG expression was observed in the peripheral blood of TMPD-treated WT mice, but not in the peripheral blood of TMPD-treated IFNAR(-/-) mice. TMPD did not induce lupus-specific autoantibodies (anti-RNP, anti-Sm, anti-double-stranded DNA) in IFNAR(-/-) mice, whereas 129Sv controls developed these specificities. Although glomerular immune complexes were present in IFNAR(-/-) mice, proteinuria and glomerular hypercellularity did not develop, whereas these features of glomerulonephritis were found in the TMPD-treated WT controls. The clinical and serologic manifestations observed in TMPD-treated mice were strongly dependent on IFNAR signaling, which is consistent with the association of increased expression of ISGs with lupus-specific autoantibodies and nephritis in humans.
Conclusion: Similar to its proposed role in human SLE, signaling via the IFNAR is central to the pathogenesis of autoantibodies and glomerulonephritis in TMPD-induced lupus. This lupus model is the first animal model shown to recapitulate the "interferon signature" in peripheral blood.
Figures
References
-
- Reeves WH, Narain S, Satoh M. Autoantibodies in systemic lupus erythematosus. In: Koopman WJ, editor. Arthritis and Allied Conditions, A Textbook of Rheumatology. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 1497–1521.
-
- Kirou KA, Lee C, George S, Louca K, Papagiannis IG, Peterson MG, et al. Coordinate overexpression of interferon-alpha-induced genes in systemic lupus erythematosus. Arthritis Rheum. 2004;50(12):3958–3967. - PubMed
-
- Zhuang H, Narain S, Sobel E, Lee PY, Nacionales DC, Kelly KM, et al. Association of anti-nucleoprotein autoantibodies with upregulation of Type I interferon-inducible gene transcripts and dendritic cell maturation in systemic lupus erythematosus. Clin Immunol. 2005;117(3):238–250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

