Association between friction and wear in diarthrodial joints lacking lubricin
- PMID: 17968947
- PMCID: PMC2688668
- DOI: 10.1002/art.22974
Association between friction and wear in diarthrodial joints lacking lubricin
Abstract
Objective: The glycoprotein lubricin (encoded by the gene Prg4) is secreted by surface chondrocytes and synovial cells, and has been shown to reduce friction in vitro. In contrast to man-made bearings, mammalian diarthrodial joints must endogenously produce friction-reducing agents. This study was undertaken to investigate whether friction is associated with wear.
Methods: The lubricating ability of synovial fluid (SF) samples from humans with genetic lubricin deficiency was tested in vitro. The coefficient of friction in the knee joints of normal and lubricin-null mice was measured ex vivo; these joints were also studied by light and electron microscopy. Atomic force microscopy was used to image and measure how lubricin reduces friction in vitro.
Results: SF lacking lubricin failed to reduce friction in the boundary mode. Joints of lubricin-null mice showed early wear and higher friction than joints from their wild-type counterparts. Lubricin self-organized and reduced the work of adhesion between apposing asperities.
Conclusion: These data show that friction is coupled with wear at the cartilage surface in vivo. They imply that acquired lubricin degradation occurring in inflammatory joint diseases predisposes the cartilage to damage. Lastly, they suggest that lubricin, or similar biomolecules, will have applications in man-made devices in which reducing friction is essential.
Figures


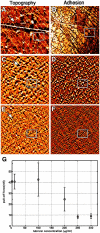
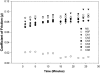
Comment in
-
Tribology: a portal to understand joint failure?Arthritis Rheum. 2007 Nov;56(11):3511-3. doi: 10.1002/art.22973. Arthritis Rheum. 2007. PMID: 17968920 No abstract available.
Similar articles
-
The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth.J Clin Invest. 2005 Mar;115(3):622-31. doi: 10.1172/JCI22263. J Clin Invest. 2005. PMID: 15719068 Free PMC article.
-
Synthesis and characterization of a lubricin mimic (mLub) to reduce friction and adhesion on the articular cartilage surface.Biomaterials. 2015 Dec;73:42-50. doi: 10.1016/j.biomaterials.2015.09.012. Epub 2015 Sep 11. Biomaterials. 2015. PMID: 26398308 Free PMC article.
-
Cyclic loading increases friction and changes cartilage surface integrity in lubricin-mutant mouse knees.Arthritis Rheum. 2012 Feb;64(2):465-73. doi: 10.1002/art.33337. Arthritis Rheum. 2012. PMID: 21905020 Free PMC article.
-
Lubrication of Articular Cartilage.Annu Rev Biomed Eng. 2016 Jul 11;18:235-58. doi: 10.1146/annurev-bioeng-081514-123305. Annu Rev Biomed Eng. 2016. PMID: 27420572 Review.
-
The biology of lubricin: near frictionless joint motion.Matrix Biol. 2014 Oct;39:17-24. doi: 10.1016/j.matbio.2014.08.008. Epub 2014 Aug 27. Matrix Biol. 2014. PMID: 25172828 Review.
Cited by
-
Gliding resistance and modifications of gliding surface of tendon: clinical perspectives.Hand Clin. 2013 May;29(2):159-66. doi: 10.1016/j.hcl.2013.02.001. Epub 2013 Mar 15. Hand Clin. 2013. PMID: 23660052 Free PMC article. Review.
-
Cartilage boundary lubricating ability of aldehyde modified proteoglycan 4 (PRG4-CHO).Osteoarthritis Cartilage. 2013 Jan;21(1):186-9. doi: 10.1016/j.joca.2012.09.016. Epub 2012 Oct 4. Osteoarthritis Cartilage. 2013. PMID: 23041437 Free PMC article. No abstract available.
-
The tribology of cartilage: Mechanisms, experimental techniques, and relevance to translational tissue engineering.Clin Biomech (Bristol). 2020 Oct;79:104880. doi: 10.1016/j.clinbiomech.2019.10.016. Epub 2019 Oct 23. Clin Biomech (Bristol). 2020. PMID: 31676140 Free PMC article. Review.
-
Lubricin: a novel potential biotherapeutic approaches for the treatment of osteoarthritis.Mol Biol Rep. 2011 Jun;38(5):2879-85. doi: 10.1007/s11033-010-9949-9. Epub 2010 Jan 23. Mol Biol Rep. 2011. PMID: 20099082 Review.
-
Excess BMP Signaling in Heterotopic Cartilage Forming in Prg4-null TMJ Discs.J Dent Res. 2016 Mar;95(3):292-301. doi: 10.1177/0022034515613508. Epub 2015 Nov 3. J Dent Res. 2016. PMID: 26534931 Free PMC article.
References
-
- Charnley J. The lubrication of animal joints. London: Institute of Mechanical Engineers Symposium on Biomechanics; 1959. pp. 12–22.
-
- McCutchen CW. The frictional properties of animal joints. Wear. 1962;5:412–5.
-
- Flannery CR, Hughes CE, Schumacher BL, Tudor D, Aydelotte MB, Kuettner KE, et al. Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Commun. 1999;254:535–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

