Improved tumor vascular function following high-dose epidermal growth factor receptor tyrosine kinase inhibitor therapy
- PMID: 17968965
- PMCID: PMC3024590
- DOI: 10.1002/jmri.21196
Improved tumor vascular function following high-dose epidermal growth factor receptor tyrosine kinase inhibitor therapy
Abstract
Purpose: To determine if inhibitors of the human growth factor receptor (HER) family can be used to enhance tumor vascular permeability and perfusion and optimize the efficacy of cytotoxic chemotherapeutics. Poor tumor vascular function limits the delivery and efficacy of cancer chemotherapeutics and HER family tyrosine kinases mediate tumor-endothelial signaling in both of these compartments.
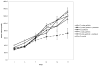
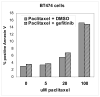
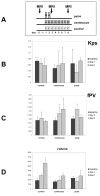
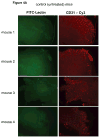
Materials and methods: BT474 human breast cancer tumors were established in mice and the biologic effects of the HER tyrosine kinase inhibitor (TKI) gefitinib on tumor vascular function was determined by dynamic contrast-enhanced MRI (DCE-MRI), and on tumor vascular architecture and perfusion by immunofluorescence microscopy.
Results: A brief dose of gefitinib enhances the antitumor activity of paclitaxel in vivo but not in cell culture, suggesting that its chemoenhancing activity involves the in vivo microenvironment. A brief high dose of gefitinib induces a decrease in endothelial transfer constant (Kps) and a concomitant increase in tumor fractional plasma volume (fPV). These changes are accompanied by a rapid reduction in tumor volume, likely due to decreased tumor edema, and modestly improved tumor vascular architecture and perfusion on microscopy.
Conclusion: These data suggest that HER family TKIs have the potential to optimize the tumor microenvironment for delivery of cytotoxic chemotherapeutics.
(c) 2007 Wiley-Liss, Inc.
Figures

Similar articles
-
AG-013736, a novel inhibitor of VEGF receptor tyrosine kinases, inhibits breast cancer growth and decreases vascular permeability as detected by dynamic contrast-enhanced magnetic resonance imaging.Magn Reson Imaging. 2007 Apr;25(3):319-27. doi: 10.1016/j.mri.2006.09.041. Epub 2007 Feb 5. Magn Reson Imaging. 2007. PMID: 17371720
-
KRN951, a highly potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, has antitumor activities and affects functional vascular properties.Cancer Res. 2006 Sep 15;66(18):9134-42. doi: 10.1158/0008-5472.CAN-05-4290. Cancer Res. 2006. PMID: 16982756
-
Anticancer effects of ZD6474, a VEGF receptor tyrosine kinase inhibitor, in gefitinib ("Iressa")-sensitive and resistant xenograft models.Cancer Sci. 2004 Dec;95(12):984-9. doi: 10.1111/j.1349-7006.2004.tb03187.x. Cancer Sci. 2004. PMID: 15596048 Free PMC article.
-
An update of the mechanisms of resistance to EGFR-tyrosine kinase inhibitors in breast cancer: Gefitinib (Iressa) -induced changes in the expression and nucleo-cytoplasmic trafficking of HER-ligands (Review).Int J Mol Med. 2007 Jul;20(1):3-10. Int J Mol Med. 2007. PMID: 17549382 Review.
-
Vascular endothelial growth factor receptor tyrosine kinase inhibitors: PTK787/ZK 222584.Semin Oncol. 2003 Jun;30(3 Suppl 6):32-8. doi: 10.1016/s0093-7754(03)00123-4. Semin Oncol. 2003. PMID: 12802793 Review.
Cited by
-
Magnetic resonance in the era of molecular imaging of cancer.Magn Reson Imaging. 2011 Jun;29(5):587-600. doi: 10.1016/j.mri.2011.02.003. Epub 2011 Apr 27. Magn Reson Imaging. 2011. PMID: 21524870 Free PMC article. Review.
-
Assessment of tumor response to tyrosine kinase inhibitors.Front Biosci (Landmark Ed). 2011 Jun 1;16(6):1996-2007. doi: 10.2741/3836. Front Biosci (Landmark Ed). 2011. PMID: 21622159 Free PMC article. Review.
-
MRI methods for evaluating the effects of tyrosine kinase inhibitor administration used to enhance chemotherapy efficiency in a breast tumor xenograft model.J Magn Reson Imaging. 2009 May;29(5):1071-9. doi: 10.1002/jmri.21737. J Magn Reson Imaging. 2009. PMID: 19388114 Free PMC article.
-
A phase I study of a 2-day lapatinib chemosensitization pulse preceding nanoparticle albumin-bound Paclitaxel for advanced solid malignancies.Clin Cancer Res. 2009 Sep 1;15(17):5569-75. doi: 10.1158/1078-0432.CCR-09-0522. Epub 2009 Aug 25. Clin Cancer Res. 2009. PMID: 19706807 Free PMC article. Clinical Trial.
-
The effect of chemotherapeutic agents on tumor vasculature in subcutaneous and orthotopic human tumor xenografts.BMC Cancer. 2015 Mar 10;15:112. doi: 10.1186/s12885-015-1091-6. BMC Cancer. 2015. PMID: 25884767 Free PMC article.
References
-
- Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–592. - PubMed
-
- Padera TP, Stoll BR, Tooredman JB, Capen D, di TE, Jain RK. Pathology: cancer cells compress intratumour vessels. Nature. 2004;427:695. - PubMed
-
- Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. - PubMed
-
- Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Research. 2004;64:3731–37336. - PubMed
-
- Miller KD, Wang M, Dickler M, Cobleigh MA, Perez EA, Shenkier TN, Davidson NE. A randomized phase III trial of paclitaxel versus paclitaxel plus bevacizumab as first-line therapy for locally recurrent or metastatic breast cancer: a trial coordinated by the Eastern Cooperative Oncology Group (E2100) Proc San Antonio Br Can Symp. 2005:3.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

