Targeting the formation of the cell wall core of M. tuberculosis
- PMID: 17970228
- PMCID: PMC4747060
- DOI: 10.2174/187152607781001808
Targeting the formation of the cell wall core of M. tuberculosis
Abstract
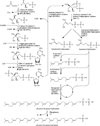
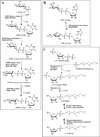
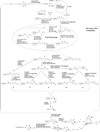
Mycobacteria have a unique cell wall, which is rich in drug targets. The cell wall core consists of a peptidoglycan layer, a mycolic acid layer, and an arabinogalactan polysaccharide connecting them. The detailed structure of the cell wall core is largely, although not completely, understood and will be presented. The biosynthetic pathways of all three components reveal significant drug targets that are the basis of present drugs and/or have potential for new drugs. These pathways will be reviewed and include enzymes involved in polyisoprene biosynthesis, soluble arabinogalactan precursor production, arabinogalactan polymerization, fatty acid synthesis, mycolate maturation, and soluble peptidoglycan precursor formation. Information relevant to targeting all these enzymes will be presented in tabular form. Selected enzymes will then be discussed in more detail. It is thus hoped this chapter will aid in the selection of targets for new drugs to combat tuberculosis.
Figures



Similar articles
-
Chapter 2: Biogenesis of the cell wall and other glycoconjugates of Mycobacterium tuberculosis.Adv Appl Microbiol. 2009;69:23-78. doi: 10.1016/S0065-2164(09)69002-X. Adv Appl Microbiol. 2009. PMID: 19729090 Free PMC article. Review.
-
Mycobacterial cell wall biosynthesis: a multifaceted antibiotic target.Parasitology. 2018 Feb;145(2):116-133. doi: 10.1017/S0031182016002377. Epub 2016 Dec 15. Parasitology. 2018. PMID: 27976597 Free PMC article. Review.
-
Arabinogalactan and lipoarabinomannan biosynthesis: structure, biogenesis and their potential as drug targets.Future Microbiol. 2012 Jan;7(1):129-47. doi: 10.2217/fmb.11.123. Future Microbiol. 2012. PMID: 22191451 Review.
-
Assembly of the Mycobacterial Cell Wall.Annu Rev Microbiol. 2015;69:405-23. doi: 10.1146/annurev-micro-091014-104121. Annu Rev Microbiol. 2015. PMID: 26488279 Review.
-
SQ109 targets MmpL3, a membrane transporter of trehalose monomycolate involved in mycolic acid donation to the cell wall core of Mycobacterium tuberculosis.Antimicrob Agents Chemother. 2012 Apr;56(4):1797-809. doi: 10.1128/AAC.05708-11. Epub 2012 Jan 17. Antimicrob Agents Chemother. 2012. PMID: 22252828 Free PMC article.
Cited by
-
Applications of Transcriptomics and Proteomics for Understanding Dormancy and Resuscitation in Mycobacterium tuberculosis.Front Microbiol. 2021 Mar 31;12:642487. doi: 10.3389/fmicb.2021.642487. eCollection 2021. Front Microbiol. 2021. PMID: 33868200 Free PMC article. Review.
-
Structural Variability of Lipoarabinomannan Modulates Innate Immune Responses within Infected Alveolar Epithelial Cells.Cells. 2022 Jan 21;11(3):361. doi: 10.3390/cells11030361. Cells. 2022. PMID: 35159170 Free PMC article.
-
Antigen 85C inhibition restricts Mycobacterium tuberculosis growth through disruption of cord factor biosynthesis.Antimicrob Agents Chemother. 2012 Apr;56(4):1735-43. doi: 10.1128/AAC.05742-11. Epub 2012 Jan 30. Antimicrob Agents Chemother. 2012. PMID: 22290959 Free PMC article.
-
Identification of amino acids involved in catalytic process of M. tuberculosis GlmU acetyltransferase.Glycoconj J. 2012 Aug;29(5-6):297-303. doi: 10.1007/s10719-012-9402-5. Epub 2012 Jun 6. Glycoconj J. 2012. PMID: 22669463
-
Exploring the chemical space of 1,2,3-triazolyl triclosan analogs for discovery of new antileishmanial chemotherapeutic agents.RSC Med Chem. 2020 Nov 5;12(1):120-128. doi: 10.1039/d0md00291g. eCollection 2021 Jan 1. RSC Med Chem. 2020. PMID: 34046604 Free PMC article.
References
-
- Projan SJ. Current Opinion in Pharmacology. 2002;2:513. - PubMed
-
- Stein J. Expert Opinion on Investigational Drugs. 2005;14:107. - PubMed
-
- Talbot GH, Bradley J, Edwards JE, Gilbert D, Scheld M, Bartlett JG. Clinical Infectious Diseases. 2006;42:657. - PubMed
-
- Mills SD. Biochemical Pharmacology. 2006;71:1096. - PubMed
-
- Draper P. In: The Biology of Mycobacteria. Ratledge C, Sanford J, editors. I. London: Academic Press; 1982. pp. 9–52.
Publication types
MeSH terms
Substances
Grants and funding
- Z01 AI000783/ImNIH/Intramural NIH HHS/United States
- R01 AI049151/AI/NIAID NIH HHS/United States
- P01 AI057836/AI/NIAID NIH HHS/United States
- AI049151/AI/NIAID NIH HHS/United States
- R01 AI033706/AI/NIAID NIH HHS/United States
- P01 AI046393/AI/NIAID NIH HHS/United States
- AI057836/AI/NIAID NIH HHS/United States
- R56 AI049151/AI/NIAID NIH HHS/United States
- AI065357-020010/AI/NIAID NIH HHS/United States
- AI33706/AI/NIAID NIH HHS/United States
- U54 AI065357/AI/NIAID NIH HHS/United States
- R01 AI097550/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

