Adult T-cell leukemia/lymphoma cells from blood and skin tumors express cytotoxic T lymphocyte-associated antigen-4 and Foxp3 but lack suppressor activity toward autologous CD8+ T cells
- PMID: 17970785
- PMCID: PMC11158631
- DOI: 10.1111/j.1349-7006.2007.00646.x
Adult T-cell leukemia/lymphoma cells from blood and skin tumors express cytotoxic T lymphocyte-associated antigen-4 and Foxp3 but lack suppressor activity toward autologous CD8+ T cells
Abstract
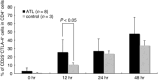
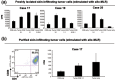
Adult T cell leukemia/lymphoma (ATL) cells share the CD4(+)CD25(+) phenotype with regulatory T (Treg) cells. However, it is still controversial whether ATL cells are Treg cells. The aim of the present study was to investigate the Treg nature of ATL cells obtained from peripheral blood and skin tumors in terms of their phenotype and function. By flow cytometry and immunohistochemistry, the expression of the Treg-associated molecule cytotoxic T lymphocyte-associated antigen (CTLA)-4 and Foxp3 was examined in freshly isolated circulating and skin-infiltrating tumor cells from 21 ATL patients with skin eruptions. The expression of CTLA-4 on freshly isolated circulating tumor cells was elevated in two of 15 patients, and Foxp3 was expressed intracytoplasmically at high levels in three of nine patients. In five of the patients examined, skin-infiltrating tumor cells bore variously elevated CTLA-4 with high Foxp3 expression. The potentiality of ATL cells as Treg cells was further addressed by stimulating ATL cells with anti-CD3/CD28 monoclonal antibodies and monitoring CTLA-4 expression. With the stimulation, even CTLA-4-low ATL cells expressed higher levels of CTLA-4 than normal CD4(+)CD25(+) cells. To study function, ATL cells isolated from blood and skin tumors were tested for their ability to suppress the proliferation of autologous CD8(+) T cells stimulated with allogeneic lymphocytes. Despite the expression of CTLA-4 and Foxp3, these tumors were incapable of suppressing the proliferation of autologous CD8(+) T cells. ATL cells are phenotypically Treg cells in at least some patients, but lack immunoregulatory functions, at least toward CD8(+) T cells.
Figures





Similar articles
-
CTLA-4 blockade following relapse of malignancy after allogeneic stem cell transplantation is associated with T cell activation but not with increased levels of T regulatory cells.Biol Blood Marrow Transplant. 2011 May;17(5):682-92. doi: 10.1016/j.bbmt.2010.08.005. Epub 2010 Aug 14. Biol Blood Marrow Transplant. 2011. PMID: 20713164 Free PMC article. Clinical Trial.
-
Acquisition of regulatory function by human CD8(+) T cells treated with anti-CD3 antibody requires TNF.Eur J Immunol. 2010 Oct;40(10):2891-901. doi: 10.1002/eji.201040485. Eur J Immunol. 2010. PMID: 21038470 Free PMC article.
-
Hypomethylation of the Treg-Specific Demethylated Region in FOXP3 Is a Hallmark of the Regulatory T-cell Subtype in Adult T-cell Leukemia.Cancer Immunol Res. 2016 Feb;4(2):136-45. doi: 10.1158/2326-6066.CIR-15-0148. Epub 2015 Dec 17. Cancer Immunol Res. 2016. PMID: 26681759
-
Comparative study of cutaneous T-cell lymphoma and adult T-cell leukemia/lymphoma. Clinical, histopathologic, and immunohistochemical analyses.Cancer. 1990 Dec 1;66(11):2380-6. doi: 10.1002/1097-0142(19901201)66:11<2380::aid-cncr2820661122>3.0.co;2-h. Cancer. 1990. PMID: 2245393 Review.
-
The role of CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) in regulatory T-cell biology.Immunol Rev. 2006 Aug;212:131-48. doi: 10.1111/j.0105-2896.2006.00419.x. Immunol Rev. 2006. PMID: 16903911 Review.
Cited by
-
HTLV-1 modulates the frequency and phenotype of FoxP3+CD4+ T cells in virus-infected individuals.Retrovirology. 2012 May 30;9:46. doi: 10.1186/1742-4690-9-46. Retrovirology. 2012. PMID: 22647666 Free PMC article.
-
Human T-lymphotropic virus type 1 (HTLV-1) and regulatory T cells in HTLV-1-associated neuroinflammatory disease.Viruses. 2011 Sep;3(9):1532-48. doi: 10.3390/v3091532. Epub 2011 Aug 25. Viruses. 2011. PMID: 21994794 Free PMC article. Review.
-
Quantifying protein abundance on single cells using split-pool sequencing on DNA-barcoded antibodies for diagnostic applications.Sci Rep. 2022 Jan 18;12(1):884. doi: 10.1038/s41598-022-04842-7. Sci Rep. 2022. PMID: 35042926 Free PMC article.
-
HTLV-1 bZIP factor induces T-cell lymphoma and systemic inflammation in vivo.PLoS Pathog. 2011 Feb 10;7(2):e1001274. doi: 10.1371/journal.ppat.1001274. PLoS Pathog. 2011. PMID: 21347344 Free PMC article.
-
Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) as an undetermined tool in tumor cells.Hum Cell. 2023 Jul;36(4):1225-1232. doi: 10.1007/s13577-023-00893-8. Epub 2023 Mar 13. Hum Cell. 2023. PMID: 36907978 Review.
References
-
- Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T‐cell leukemia: clinical and hematologic features of 16 cases. Blood 1977; 50: 481–92. - PubMed
-
- Blattner WA. Human retroviruses: their role in cancer. Proc Assoc Am Physicians 1999; 111: 563–72. - PubMed
-
- Edlich RF, Arnette JA, Williams FM. Global epidemic of human T‐cell lymphotropic virus type‐I (HTLV‐I). J Emerg Med 2000; 18: 109–19. - PubMed
-
- Shimoyama M, Members of the Lymphoma Study Group (1984–87) . Diagnostic criteria and classification of clinical subtypes of adult T‐cell leukaemia‐lymphoma: a report from Lymphoma Study Group 1984–87. Br J Haematol 1991; 79: 428–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

