Phase III study to evaluate the use of high-dose chemotherapy as consolidation of treatment for high-risk postoperative breast cancer: Japan Clinical Oncology Group study, JCOG 9208
- PMID: 17970786
- PMCID: PMC11159025
- DOI: 10.1111/j.1349-7006.2007.00639.x
Phase III study to evaluate the use of high-dose chemotherapy as consolidation of treatment for high-risk postoperative breast cancer: Japan Clinical Oncology Group study, JCOG 9208
Abstract
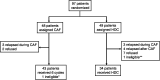
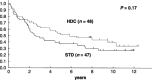
A randomized controlled trial was conducted to evaluate the efficacy of high-dose chemotherapy (HDC) as consolidation of the treatment of high-risk postoperative breast cancer. Patients under 56 years of age with stage I to IIIB breast cancer involving 10 or more axillary lymph nodes were eligible. The primary endpoint was relapse-free survival (RFS). Between May 1993 and March 1999, 97 patients were enrolled, and two patients became ineligible. The median age of the 97 patients was 46 years (range 27-55 years), and 72 (74%) were premenopausal. The median number of involved axillary nodes was 16 (range 10-49). All patients had undergone a radical mastectomy. Major characteristics were well balanced between the treatment arms. Forty-eight patients in the standard-dose (STD) arm received six courses of cyclophosphamide, doxorubicin, and 5-fluorouracil followed by tamoxifen. Forty-nine patients were assigned to undergo HDC with cyclophosphamide and thiotepa after six courses of cyclophosphamide, doxorubicin, and 5-fluorouracil followed by tamoxifen; however, 15 of these patients (31%) did not undergo HDC. HDC was well tolerated without any treatment-related mortality. At a median follow-up of 63 months, the 5-year RFS of 47 eligible patients in the STD arm and 48 eligible patients in the HDC arm was 37% and 52% on an intent-to-treat basis, respectively (P = 0.17). Five-year overall survival of all randomized patients was 62% for the STD arm and 63% for the HDC arm (P = 0.78). Although the prespecified values of the two arms were not so accurate as to allow detection of the observed difference, no advantage of HDC was observed in terms of RFS or overall survival.
Figures


References
-
- Skipper HE. Dose intensity versus total dose chemotherapy: an experimental basis. In: DeVita VT, Hellman S, Rosenberg SA, eds. Important Advances in Oncology. Philadelphia, PA: Lippincott, 1990: 43–64. - PubMed
-
- Philip T, Guglielmi C, Hagenbeek A et al . Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy‐sensitive non‐Hodgkin's lymphoma. N Engl J Med 1995; 333: 1540–5. - PubMed
-
- Attal M, Harousseau J‐L, Stoppa A‐M, Sotto J‐J et al ., for The Intergroupe Français du Myélome . A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. N Engl J Med 1996; 335: 91–9. - PubMed
-
- Child JA, Morgan GJ, Davies FE et al ., for the Medical Research Council Adult Leukaemia Working Party . High‐dose chemotherapy with hematopoietic stem‐cell rescue for multiple myeloma. N Engl J Med 2003; 348: 1875–83. - PubMed
-
- Clarke M, Collins R, Darby S, Davies C et al ., Early Breast Cancer Trialists’ Collaborative Group (EBCTCG ). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15‐year survival: an overview of the randomised trials. Lancet 2005; 365: 1687–717. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials

