Characterization of spectral FRET imaging microscopy for monitoring nuclear protein interactions
- PMID: 17970914
- PMCID: PMC2874973
- DOI: 10.1111/j.1365-2818.2007.01838.x
Characterization of spectral FRET imaging microscopy for monitoring nuclear protein interactions
Abstract
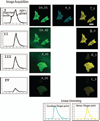
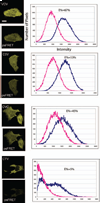
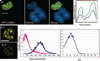
The spectral processed Förster resonance energy transfer (psFRET) imaging method provides an effective and fast method for measuring protein-protein interactions in living specimens. The commercially available linear unmixing algorithms efficiently remove the contribution of donor spectral bleedthrough to the FRET signal. However, the acceptor contribution to spectral bleedthrough in the FRET image cannot be similarly removed, since the acceptor spectrum is identical to the FRET spectrum. Here, we describe the development of a computer algorithm that measures and removes the contaminating ASBT signal in the sFRET image. The new method is characterized in living cells that expressed FRET standards in which the donor and acceptor fluorescent proteins are tethered by amino acid linkers of specific lengths. The method is then used to detect the homo-dimerization of a transcription factor in the nucleus of living cells, and then to measure the interactions of that protein with a second transcription factor.
Figures




Similar articles
-
Additional correction for energy transfer efficiency calculation in filter-based Forster resonance energy transfer microscopy for more accurate results.J Biomed Opt. 2010 Mar-Apr;15(2):020513. doi: 10.1117/1.3407655. J Biomed Opt. 2010. PMID: 20459222 Free PMC article.
-
Intensity range based quantitative FRET data analysis to localize protein molecules in live cell nuclei.J Fluoresc. 2006 Jan;16(1):95-104. doi: 10.1007/s10895-005-0024-1. Epub 2006 Jan 6. J Fluoresc. 2006. PMID: 16397825
-
Quantitative Förster resonance energy transfer efficiency measurements using simultaneous spectral unmixing of excitation and emission spectra.J Biomed Opt. 2013 Feb;18(2):26024. doi: 10.1117/1.JBO.18.2.026024. J Biomed Opt. 2013. PMID: 23423332
-
Monitoring protein interactions in living cells with fluorescence lifetime imaging microscopy.Methods Enzymol. 2012;504:371-91. doi: 10.1016/B978-0-12-391857-4.00019-7. Methods Enzymol. 2012. PMID: 22264545 Free PMC article. Review.
-
Protein localization in living cells and tissues using FRET and FLIM.Differentiation. 2003 Dec;71(9-10):528-41. doi: 10.1111/j.1432-0436.2003.07109007.x. Differentiation. 2003. PMID: 14686950 Review.
Cited by
-
Wide-field microscopic FRET imaging using simultaneous spectral unmixing of excitation and emission spectra.Opt Express. 2016 Jul 11;24(14):16037-51. doi: 10.1364/OE.24.016037. Opt Express. 2016. PMID: 27410873 Free PMC article.
-
Additional correction for energy transfer efficiency calculation in filter-based Forster resonance energy transfer microscopy for more accurate results.J Biomed Opt. 2010 Mar-Apr;15(2):020513. doi: 10.1117/1.3407655. J Biomed Opt. 2010. PMID: 20459222 Free PMC article.
-
Clustering of phosphatase RPTPα promotes Src signaling and the arthritogenic action of synovial fibroblasts.Sci Signal. 2023 Jul 4;16(792):eabn8668. doi: 10.1126/scisignal.abn8668. Epub 2023 Jul 4. Sci Signal. 2023. PMID: 37402225 Free PMC article.
-
NKX2-5 mutations causative for congenital heart disease retain functionality and are directed to hundreds of targets.Elife. 2015 Jul 6;4:e06942. doi: 10.7554/eLife.06942. Elife. 2015. PMID: 26146939 Free PMC article.
-
Förster resonance energy transfer photoacoustic microscopy.J Biomed Opt. 2012 Aug;17(8):086007. doi: 10.1117/1.JBO.17.8.086007. J Biomed Opt. 2012. PMID: 23224194 Free PMC article.
References
-
- Centonze VE, Sun M, Masuda A, Gerritsen H, Herman B. Fluorescence resonance energy transfer imaging microscopy. Meth. Enzymol. 2003;360:542–560. - PubMed
-
- Chen Y, Mills JD, Periasamy A. Protein interactions in cells and tissues using FLIM and FRET. Differentiation. 2003;71:528–541. - PubMed
-
- Chen Y, Periasamy A. Characterization of two-photon excitation fluorescence lifetime imaging microscopy for protein localization. Microsc. Res. Tech. 2004;63:72–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

