Blockade of endogenous B7-H1 suppresses antibacterial protection after primary Listeria monocytogenes infection
- PMID: 17971153
- PMCID: PMC2433284
- DOI: 10.1111/j.1365-2567.2007.02708.x
Blockade of endogenous B7-H1 suppresses antibacterial protection after primary Listeria monocytogenes infection
Abstract
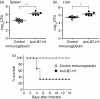
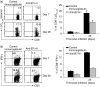
B7-H1 (also known as CD274 and PD-L1) is a cosignalling molecule regulating T-cell immunity positively or negatively in vivo. However, little is known about the role of endogenous B7-H1 in bacterial infection. We found that B7-H1 expression was up-regulated in various cell populations including CD4+ and CD8+ T cells, natural killer (NK) cells and macrophages following Listeria monocytogenes infection. Administration of the antagonistic B7-H1 monoclonal antibody resulted in a significant increase in mortality in mice infected with a lethal dose of L. monocytogenes compared with mice given the control immunoglobulin. In vivo blockade of B7-H1 greatly inhibited the production of tumour necrosis factor (TNF)-alpha and nitric oxide, key effector molecules responsible for intracellular killing by macrophages. B7-H1 blockade also suppressed the expression of granzyme B and interferon (IFN)-gamma by NK cells. Interestingly, blocking of endogenous B7-H1 selectively inhibited CD8+ T cells rather than CD4+ T cells in response to L. monocytogenes infection, as evidenced by the reduction of IFN-gamma production and the expression of effector surface markers including CD62L(int/low) and CD44(high) in CD8+ T cells from mice given anti-B7-H1 monoclonal antibody. In addition, we found that the proliferation of listeriolysin-O (LLO)-specific and IFN-gamma-producing L. monocytogenes-reactive CD8+ T cells was significantly decreased not only in the effector phase but also in the memory phase in the presence of anti-B7-H1 antibody. Our findings thus suggest that endogenous B7-H1 can provide positive costimulatory signals for innate and adaptive immunity leading to protection against intracellular bacterial infection.
Figures


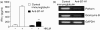

References
-
- Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992;7:521–31. - PubMed
-
- Mombaerts P, Arnoldi J, Russ F, Tonegawa S, Kaufmann SH. Different roles of αβ and γδT cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:53–6. - PubMed
-
- Edelson BT, Unanue ER. Immunity to Listeria infection. Curr Opin Immunol. 2000;12:425–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

