Identification of palmitoylated mitochondrial proteins using a bio-orthogonal azido-palmitate analogue
- PMID: 17971398
- PMCID: PMC2860959
- DOI: 10.1096/fj.07-9199com
Identification of palmitoylated mitochondrial proteins using a bio-orthogonal azido-palmitate analogue
Abstract
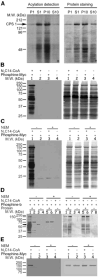
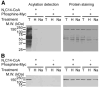
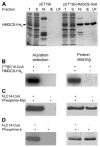
Increased levels of circulating saturated free fatty acids, such as palmitate, have been implicated in the etiology of type II diabetes and cancer. In addition to being a constituent of glycerolipids and a source of energy, palmitate also covalently attaches to numerous cellular proteins via a process named palmitoylation. Recognized for its roles in membrane tethering, cellular signaling, and protein trafficking, palmitoylation is also emerging as a potential regulator of metabolism. Indeed, we showed previously that the acylation of two mitochondrial proteins at their active site cysteine residues result in their inhibition. Herein, we sought to identify other palmitoylated proteins in mitochondria using a nonradioactive bio-orthogonal azido-palmitate analog that can be selectively derivatized with various tagged triarylphosphines. Our results show that, like palmitate, incorporation of azido-palmitate occurred on mitochondrial proteins via thioester bonds at sites that could be competed out by palmitoyl-CoA. Using this method, we identified 21 putative palmitoylated proteins in the rat liver mitochondrial matrix, a compartment not recognized for its content in palmitoylated proteins, and confirmed the palmitoylation of newly identified mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase. We postulate that covalent modification and perhaps inhibition of various mitochondrial enzymes by palmitoyl-CoA could lead to the metabolic impairments found in obesity-related diseases.
Figures
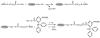


References
-
- Wilcox C, Hu JS, Olson EN. Acylation of proteins with myristic acid occurs cotranslationally. Science. 1987;238:1275–1278. - PubMed
-
- Utsumi T, Sakurai N, Nakano K, Ishisaka R. C-terminal 15 kDa fragment of cytoskeletal actin is posttranslationally N-myristoylated upon caspase-mediated cleavage and targeted to mitochondria. FEBS Lett. 2003;539:37–44. - PubMed
-
- Zha J, Weiler S, Oh KJ, Wei MC, Korsmeyer SJ. Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science. 2000;290:1761–1765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

