Berberine improves glucose metabolism through induction of glycolysis
- PMID: 17971514
- PMCID: PMC2464622
- DOI: 10.1152/ajpendo.00211.2007
Berberine improves glucose metabolism through induction of glycolysis
Abstract
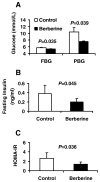
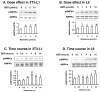
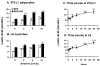
Berberine, a botanical alkaloid used to control blood glucose in type 2 diabetes in China, has recently been reported to activate AMPK. However, it is not clear how AMPK is activated by berberine. In this study, activity and action mechanism of berberine were investigated in vivo and in vitro. In dietary obese rats, berberine increased insulin sensitivity after 5-wk administration. Fasting insulin and HOMA-IR were decreased by 46 and 48%, respectively, in the rats. In cell lines including 3T3-L1 adipocytes, L6 myotubes, C2C12 myotubes, and H4IIE hepatocytes, berberine was found to increase glucose consumption, 2-deoxyglucose uptake, and to a less degree 3-O-methylglucose (3-OMG) uptake independently of insulin. The insulin-induced glucose uptake was enhanced by berberine in the absence of change in IRS-1 (Ser307/312), Akt, p70 S6, and ERK phosphorylation. AMPK phosphorylation was increased by berberine at 0.5 h, and the increase remained for > or =16 h. Aerobic and anaerobic respiration were determined to understand the mechanism of berberine action. The long-lasting phosphorylation of AMPK was associated with persistent elevation in AMP/ATP ratio and reduction in oxygen consumption. An increase in glycolysis was observed with a rise in lactic acid production. Berberine exhibited no cytotoxicity, and it protected plasma membrane in L6 myotubes in the cell culture. These results suggest that berberine enhances glucose metabolism by stimulation of glycolysis, which is related to inhibition of glucose oxidation in mitochondria. Berberine-induced AMPK activation is likely a consequence of mitochondria inhibition that increases the AMP/ATP ratio.
Figures






References
-
- Barreto MC, Pinto RE, Arrabaca JD, Pavao ML. Inhibition of mouse liver respiration by Chelidonium majus isoquinoline alkaloids. Toxicol Lett. 2003;146:37–47. - PubMed
-
- Brusq JM, Ancellin N, Grondin P, Guillard R, Martin S, Saintillan Y, Issandou M. Inhibition of lipid synthesis through activation of AMP-kinase: An additional mechanism for the hypolipidemic effects of Berberine. J Lipid Res. 2006 - PubMed
-
- Cheng Z, Pang T, Gu M, Gao AH, Xie CM, Li JY, Nan FJ, Li J. Berberine-stimulated glucose uptake in L6 myotubes involves both AMPK and p38 MAPK. Biochim Biophys Acta. 2006;1760:1682–1689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

