A randomised controlled trial to assess the efficacy of dihydroartemisinin-piperaquine for the treatment of uncomplicated falciparum malaria in Peru
- PMID: 17971864
- PMCID: PMC2040506
- DOI: 10.1371/journal.pone.0001101
A randomised controlled trial to assess the efficacy of dihydroartemisinin-piperaquine for the treatment of uncomplicated falciparum malaria in Peru
Abstract
Background: Multi-drug resistant falciparum malaria is an important health problem in the Peruvian Amazon region. We carried out a randomised open label clinical trial comparing mefloquine-artesunate, the current first line treatment in this region, with dihydroartemisinin-piperaquine.
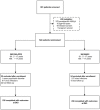
Methods and findings: Between July 2003 and July 2005, 522 patients with P. falciparum uncomplicated malaria were recruited, randomized (260 with mefloquine-artesunate and 262 with dihydroartemisinin-piperaquine), treated and followed up for 63 days. PCR-adjusted adequate clinical and parasitological response, estimated by Kaplan Meier survival and Per Protocol analysis, was extremely high for both drugs (99.6% for mefloquine-artesunate and 98.4% and for dihydroartemisinin-piperaquine) (RR: 0.99, 95%CI [0.97-1.01], Fisher Exact p = 0.21). All recrudescences were late parasitological failures. Overall, gametocytes were cleared faster in the mefloquine-artesunate group (28 vs 35 days) and new gametocytes tended to appear more frequently in patients treated with dihydroartemisinin-piperaquine (day 7: 8 (3.6%) vs 2 (0.9%), RR: 3.84, 95%CI [0.82-17.87]). Adverse events such as anxiety and insomnia were significantly more frequent in the mefloquine-artesunate group, both in adults and children.
Conclusion: Dihydroartemisinin-piperaquine is as effective as mefloquine-artesunate in treating uncomplicated P. falciparum malaria but it is better tolerated and more affordable than mefloquine-artesunate (US$1.0 versus US$18.65 on the local market). Therefore, it should be considered as a potential candidate for the first line treatment of P. falciparum malaria in Peru.
Trial registration: ClinicalTrials.gov NCT00373607.
Conflict of interest statement
Figures
References
-
- Navitsky RC, Witzig RS, Quintana J, Rios M, Aramburú JS, et al. In vivo resistance of Plasmodium falciparum to pyrimethamine/sulfadoxine in children of the Amazon region of Peru. Am J Trop Med Hyg. 1997;57(Suppl):229.
-
- Chauca H, Quintana J. Evaluación in vivo de la respuesta de Plasmodium falciparum a la cloroquina en foco carretera Yurimaguas-Tarapoto (Región Loreto). Rev Per Epi. 1993;6:34–9.
-
- Ministry of Health, Peru. Diresa Loreto, DIGEMID, DGSP, Proyecto VIGIA. Results of an intensive surveillance of adverse events related to the first line treatment (Mefloquine-Artesunate) for P. falciparum malaria in the Peruvian Amazon. 2004 Interne Report.
-
- Ruebush T, Neyra D, Cabezas C. El proceso de la adecuación y cambio en la política de tratamiento de la malaria por P. falciparum en el Perú, 1990–2001. Rev Peru Med Exp Salud Pública. 2003;20(3):162–171.
-
- Denis M, Davis T, Hewitt S, Incardona S, Nimol K, et al. Efficacy and safety of dihydroartemisinin-piperaquine (Artekin) in Cambodian children and adults with uncomplicated falciparum malaria. Clin Infect Dis. 2002;35:1469–76. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical